D64600
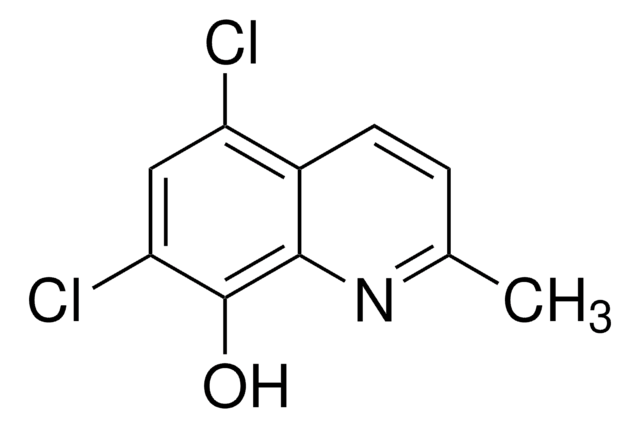
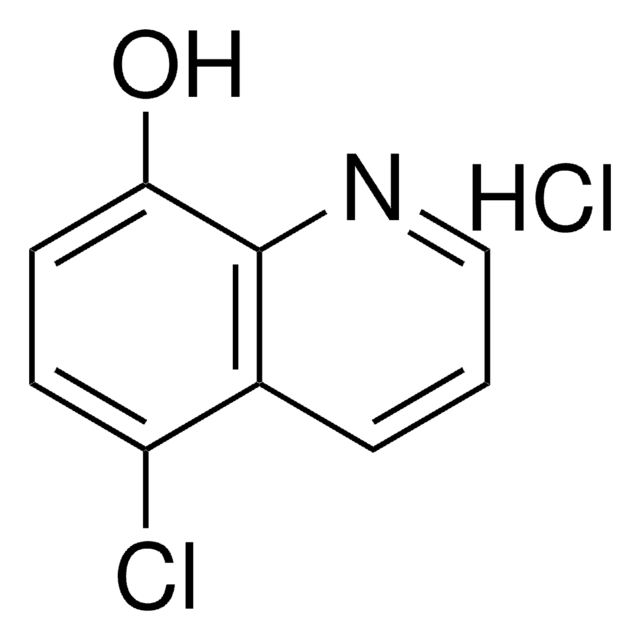
5,7-Dichloro-8-quinolinol
99%
Synonym(s):
5,7-Dichloro-8-hydroxyquinoline, Chloroxine
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C9H5Cl2NO
CAS Number:
Molecular Weight:
214.05
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
form
powder
mp
178-180 °C (lit.)
SMILES string
Oc1c(Cl)cc(Cl)c2cccnc12
InChI
1S/C9H5Cl2NO/c10-6-4-7(11)9(13)8-5(6)2-1-3-12-8/h1-4,13H
InChI key
WDFKMLRRRCGAKS-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Dam. 1 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Effects of chlorhexidine, iodine, and 5,7-dichloro-8-hydroxyquinoline on the bacterial composition of rat plaque in vivo.
M J Schaeken et al.
Caries research, 18(5), 440-446 (1984-01-01)
Miriam Aide Castillo Rodríguez et al.
Journal of pharmaceutical and biomedical analysis, 166, 113-118 (2019-01-15)
A new, rapid, simple and specific method to determine 5-chloro 8-hydroxyquinoline (5-HQ) and 5,7-dichloro 8-hydroxyquinoline (5,7-HQ) stability in swine feed was optimized and validated. A system consisting of an ACQUITY UPLC BEH C18 column (1.7 μm, 2.1 mm × 100 mm), a mobile phase of
Luying Xun et al.
The Journal of biological chemistry, 279(8), 6696-6700 (2003-12-10)
Ralstonia eutropha JMP134 2,4,6-trichlorophenol (2,4,6-TCP) 4-monooxygenase catalyzes sequential dechlorinations of 2,4,6-TCP to 6-chlorohydroxyquinol. Although 2,6-dichlorohydroxyquinol is a logical metabolic intermediate, the enzyme hardly uses it as a substrate, implying it may not be a true intermediate. Evidence is provided to
[Clinical evaluation on the usefulness of 5,7-dichloro-8-hydroxyquinoline (chloroxine) in association with betamethasone 17-benzoate in the topical treatment of infected cortisone-sensitive dermopathies].
M Negosanti et al.
Giornale italiano di dermatologia e venereologia : organo ufficiale, Societa italiana di dermatologia e sifilografia, 120(2), XVII-XXIII (1985-03-01)
Dmytro Havrylyuk et al.
European journal of medicinal chemistry, 156, 790-799 (2018-07-29)
8-Hydroxyquinolines (HQ), including clioquinol, possess cytotoxic properties and are widely used as ligands for metal-based anticancer drug research. The number and identity of substituents on the HQ can have a profound effect on activity for a variety of inorganic compounds.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service