C47000
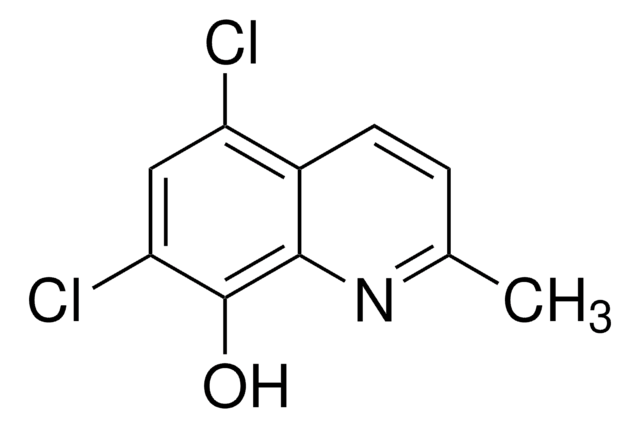
5-Chloro-8-quinolinol
95%
Synonym(s):
5-Chloro-8-hydroxyquinoline
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C9H6ClNO
CAS Number:
Molecular Weight:
179.60
Beilstein:
5289
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
95%
form
crystals
mp
122-124 °C (lit.)
SMILES string
Oc1ccc(Cl)c2cccnc12
InChI
1S/C9H6ClNO/c10-7-3-4-8(12)9-6(7)2-1-5-11-9/h1-5,12H
InChI key
CTQMJYWDVABFRZ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Aquatic Acute 1 - Aquatic Chronic 1 - Eye Irrit. 2 - Skin Sens. 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
V Arjunan et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 72(4), 783-788 (2008-12-30)
The Fourier transform infrared (FTIR) and FT-Raman spectra of 7-bromo-5-chloro-8-hydroxyquinoline (BCHQ) have been measured in the range 4000-400 and 4000-100cm(-1), respectively. Complete vibrational assignment and analysis of the fundamental modes of the compound were carried out using the observed FTIR
E J Wojtowicz
Journal of pharmaceutical sciences, 73(10), 1430-1433 (1984-10-01)
A reverse-phase high-performance liquid chromatographic (HPLC) method was developed for determining iodochlorhydroxyquin, 5,7-dichloro-8-hydroxyquinoline, and 5,7-diiodo-8-hydroxyquinoline in creams, ointments, shampoos, tablets, and bulk drugs. A column packed with 10-micron phenyl-silica and a mobile phase of 0.001 M NiCl2 in acetonitrile-methanol-water (30:20:50)
Poonpilas Hongmanee et al.
Antimicrobial agents and chemotherapy, 51(3), 1105-1106 (2006-12-21)
The in vitro activities of cloxyquin (5-chloroquinolin-8-ol) against 9 standard strains and 150 clinical isolates of Mycobacterium tuberculosis were studied. The MICs ranged from 0.062 to 0.25 microg/ml. The MIC(50) and MIC(90) were 0.125 and 0.25 microg/ml, respectively. These indicate
Oleg V Larionov et al.
Organic letters, 16(3), 864-867 (2014-01-15)
A one-step transformation of heterocyclic N-oxides to 2-alkyl-, aryl-, and alkenyl-substituted N-heterocycles is described. The success of this broad-scope methodology hinges on the combination of copper catalysis and activation by lithium fluoride or magnesium chloride. The utility of this method
Marcos N Soares et al.
Materials science & engineering. C, Materials for biological applications, 33(4), 2213-2220 (2013-03-19)
A innovative 5-Cl-8-oxyquinolinepropoxycalix[4]arene ligand (2) have been prepared, exhibiting, at room temperature, blue fluorescent light emission and resulting in shift band to green fluorescent light (fluorescence mode) in the presence of coordinated Eu(III) and Tb(III) ions. Terbium complex presented phosphorescence
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service