C86006
Crotyl alcohol, mixture of cis and trans
96%
Synonym(s):
2-Buten-1-ol
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
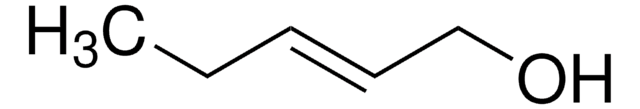
CH3CH=CHCH2OH
CAS Number:
Molecular Weight:
72.11
Beilstein:
1361395
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
96%
form
liquid
refractive index
n20/D 1.427 (lit.)
bp
121-122 °C (lit.)
density
0.845 g/mL at 25 °C (lit.)
SMILES string
C\C=C\CO
InChI
1S/C4H8O/c1-2-3-4-5/h2-3,5H,4H2,1H3/b3-2+
InChI key
WCASXYBKJHWFMY-NSCUHMNNSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
Crotyl alcohol can be used as a starting material in the synthesis of antitumor agents such as 14-azacamptothecin and 10, 11-methylenedioxy-14-azacamptothecin. It can also be used as a precursor in the total synthesis of discodermolide.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Oral - Flam. Liq. 3
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
93.2 °F - closed cup
Flash Point(C)
34 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Synthesis of optically active β-alkyl aspartate via [3, 3] sigmatropic rearrangement of α-acyloxytrialkylsilane.
Sakaguchi K, et al.
Tetrahedron Letters, 45(30), 5869-5872 (2004)
Synthesis of 14-azacamptothecin, a water-soluble topoisomerase I poison.
Rahier N J, et al.
Organic Letters, 7(5), 835-837 (2005)
Darby R Schmidt et al.
Organic letters, 5(19), 3535-3537 (2003-09-12)
[structure: see text] A synthesis of the C(15)-C(30) fragment of Dolabelides A and B has been achieved. The recently developed asymmetric silane alcoholysis and tandem silylformylation-crotylsilylation reactions were used as the key steps to establish the C(23)-C(27) 1,5-syn-diol. In addition
Frank R Fontaine et al.
Chemical research in toxicology, 15(8), 1051-1058 (2002-08-20)
Recent confirmation that the toxic unsaturated aldehyde crotonaldehyde (CA) contributes to protein damage during lipid peroxidation confers interest on the molecular actions of this substance. However, since a plethora of structurally related aldehydes form during membrane oxidation, clarifying the toxicological
Evaluation of the role of acetaldehyde in the actions of ethanol on gluconeogenesis by comparison with the effects of crotonol and crotonaldehyde.
A I Cederbaum et al.
Alcoholism, clinical and experimental research, 6(1), 100-109 (1982-01-01)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service