All Photos(1)
About This Item
Empirical Formula (Hill Notation):
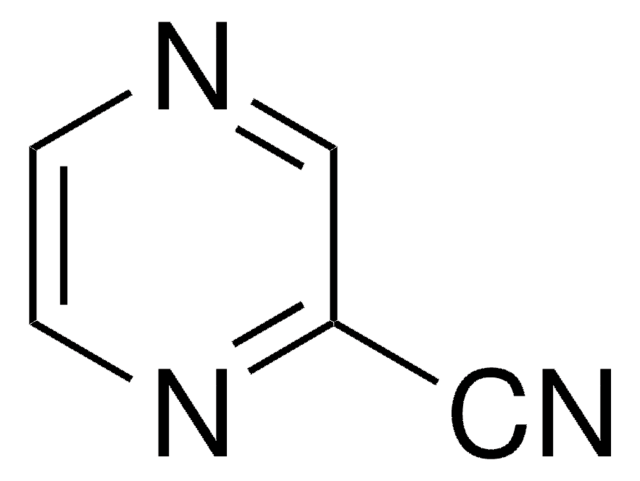
C4H5N3
CAS Number:
Molecular Weight:
95.10
Beilstein:
107025
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
crystals
mp
118-120 °C (lit.)
SMILES string
Nc1cnccn1
InChI
1S/C4H5N3/c5-4-3-6-1-2-7-4/h1-3H,(H2,5,7)
InChI key
XFTQRUTUGRCSGO-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Substrate in a four-component synthesis of imidazolidines.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Synthetic Communications, 37, 247-247 (2007)
W H Lunn et al.
Xenobiotica; the fate of foreign compounds in biological systems, 22(11), 1239-1241 (1992-11-01)
1. The compound 2-aminopyrazine was given by oral gavage to normal rats and their urine collected. 2. A mercapturic acid containing the 2-aminopyrazine moiety was isolated from this urine. This represents the first example of this type of a metabolite
J F Cavalier et al.
Bioorganic & medicinal chemistry, 9(4), 1037-1044 (2001-05-17)
A series of 5-aryl- and 3,5-bis-aryl-2-amino-1,4-pyrazine derivatives 4 and 6, and related imidazolopyrazinones 7, has been synthesized, the aryl groups of which are catechol and/or phenol substituents. These compounds, tested against human keratinocyte cells stressed by UVB irradiation, showed high
Frédéric De Wael et al.
European journal of medicinal chemistry, 45(9), 3564-3574 (2010-06-24)
Based on the imidazo-[1,2-a]-pyrazin-3-(7H)-one scaffold, a dual action prodrug has been designed for combining antioxidant and anti-inflammatory activities, possibly unmasked upon oxidation. The construction of the target-molecule requires two building blocks, namely a 2-amino-1,4-pyrazine and a 2-ketoaldehyde. Attempts to synthesize
Anna Eriksson et al.
Biochemical pharmacology, 80(10), 1507-1516 (2010-08-14)
Aberrant signal transduction by mutant or overexpressed protein kinases has emerged as a promising target for treatment of acute myeloid leukemia (AML). We here present a novel low molecular weight kinase inhibitor, AKN-032, targeting the FMS-like tyrosine kinase 3 (FLT3)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service