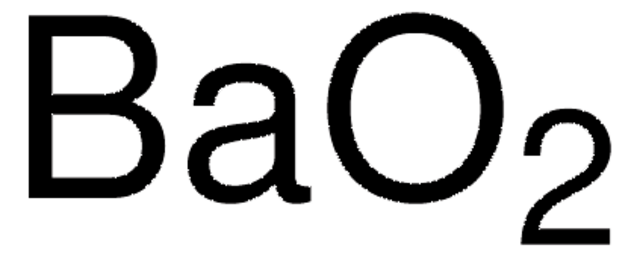72262
Nickel (IV) oxide
technical, oxidizing agent, ~30% active peroxide basis
Synonym(s):
Nickel dioxide, Nickel oxide
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
NiO2
CAS Number:
Molecular Weight:
90.69
EC Number:
MDL number:
UNSPSC Code:
12352000
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
grade
oxidizing agent
technical
Quality Level
form
powder
reaction suitability
core: nickel
reagent type: catalyst
reagent type: oxidant
concentration
~30% (active peroxide)
SMILES string
O=[Ni]O[Ni]=O
InChI
1S/2Ni.3O
InChI key
PZFKDUMHDHEBLD-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Catalyst for:
Used to study differences in reactivity trends for carbon monoxide catalyzed oxidation caused by anionic transition metal oxide clusters
Reagent:
- One-dimensional oxide-metal hybrid structures and site-specific enhanced reactivity for CO oxidation
- Microwave induced catalytic degradation of crystal violet
Used to study differences in reactivity trends for carbon monoxide catalyzed oxidation caused by anionic transition metal oxide clusters
Reagent:
- For solvent-free oxidation of alcohols†
- For chemoselective oxidation of organic compounds using inductive heating
- To improve the synthesis of methoxy cephalosporin′s intermediate
Other Notes
Oxidant for the transformation of alcohols into acids and hydrazones into diazoalkanes
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Aquatic Chronic 4 - Carc. 1A Inhalation - Ox. Sol. 2 - Skin Sens. 1 - STOT RE 1 Inhalation
Storage Class Code
5.1B - Oxidizing hazardous materials
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Javier Jiménez-Lamana et al.
Nanomaterials (Basel, Switzerland), 10(5) (2020-05-28)
Although nickel allergy and carcinogenicity are well known, their molecular mechanisms are still uncertain, thus demanding studies at the molecular level. The nickel carcinogenicity is known to be dependent on the chemical form of nickel, since only certain nickel compounds
Shadabul Haque et al.
Journal of controlled release : official journal of the Controlled Release Society, 307, 32-43 (2019-06-04)
The development of inhalable 'nanomedicines' based on biocompatible lipids and polymers is attracting increasing interest worldwide. Our understanding of how pulmonary inflammation impacts on lung distribution and clearance kinetics however, is limited. Similarly, there is limited information on how the
K. Nakagawa et al.
The Journal of Organic Chemistry, 27, 1597-1597 (1962)
Rut Sanchis et al.
Materials (Basel, Switzerland), 12(18) (2019-09-12)
Different nickel catalysts have been tested for the transformation of levulinic acid into γ-valerolactone using an easy hydrothermal method, taking advantage of the properties of the high temperature water. A metallic nickel catalyst derived from NiO synthesized by a nanocasting
Chien-Chen Diao et al.
Nanomaterials (Basel, Switzerland), 10(4) (2020-04-03)
In this study, a p-type 2 at% lithium-doped nickel oxide (abbreviation L2NiO) solution was prepared using Ni(NO3)2·6H2O, and LiNO3·L2NiO thin films were deposited using an atomizer by spraying the L2NiO solution onto a glass substrate. The sprayed specimen was heated
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service