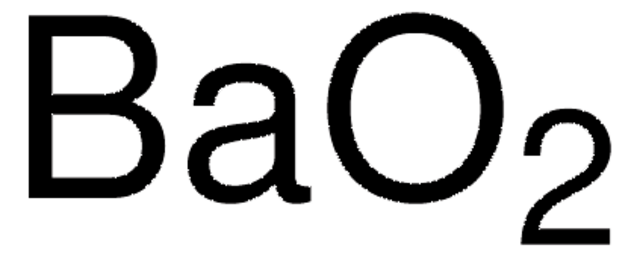All Photos(1)
About This Item
Linear Formula:
ZnO2
CAS Number:
Molecular Weight:
97.39
MDL number:
UNSPSC Code:
12352303
PubChem Substance ID:
NACRES:
NA.23
Recommended Products
form
powder
Quality Level
reaction suitability
reagent type: catalyst
core: zinc
reagent type: oxidant
concentration
50-60%
mp
212 °C (dec.) (lit.)
density
1.57 g/mL at 25 °C (lit.)
SMILES string
[Zn++].[O-][O-]
InChI
1S/O2.Zn/c1-2;/q-2;+2
InChI key
IPTOGCUGCFHDSS-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Solutions of zinc peroxide can be prepared from zinc hydroxide precursors, the sol can be subsequently used to prepare zinc nanoparticles.
Other Notes
Remainder ZnO
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Aquatic Acute 1 - Aquatic Chronic 1 - Eye Irrit. 2 - Ox. Sol. 1 - Skin Irrit. 2
Storage Class Code
5.1A - Strongly oxidizing hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Synthesis of ZnO Nanoparticles by Decomposition of Zinc Peroxide
Uekawa N, et al.
Chemistry Letters (Jpn), 7, 606-607 (2001)
Nonstoichiometric properties of zinc oxide nanoparticles prepared by decomposition of zinc peroxide.
Uekawa N, et al.
Physical Chemistry Chemical Physics, 5(5), 929-934 (2003)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service