All Photos(1)
About This Item
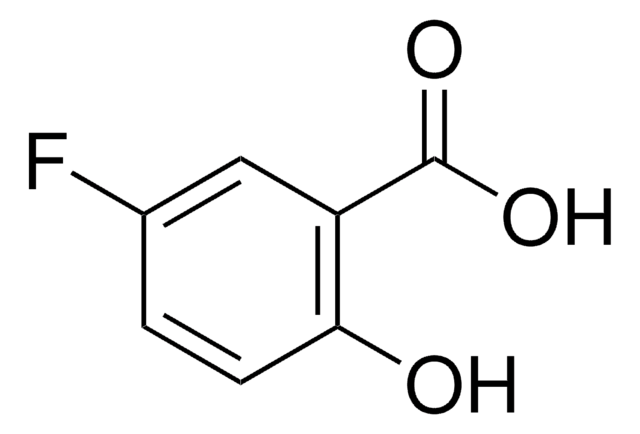
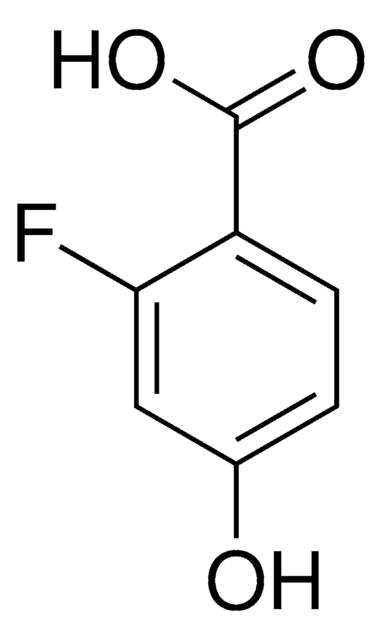
Linear Formula:
FC6H3(OH)CO2H
CAS Number:
Molecular Weight:
156.11
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
96%
mp
170 °C (dec.) (lit.)
functional group
carboxylic acid
fluoro
SMILES string
OC(=O)c1ccc(F)cc1O
InChI
1S/C7H5FO3/c8-4-1-2-5(7(10)11)6(9)3-4/h1-3,9H,(H,10,11)
InChI key
TTZOLDXHOCCNMF-UHFFFAOYSA-N
General description
4-Fluorosalicylic acid can be synthesized from the following compounds:
- 4-fluorotoluene
- m-fluorophenol
- 4-aminosalicylic acid
Application
4-Fluorosalicylic acid may be used to synthesize 4-fluorogentisic acid.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Effect of intramolecular hydrogen bonding on the relative acidities of substituted salicylic acids in benzene solution.
Dunn GE and Penner TL.
Canadian Journal of Chemistry, 45(14), 1699-1706 (1967)
Fluorosalicylohydroxamic acids.
Ostaszynski A, et al.
Bulletin de l'Academie Polonaise des Sciences. Serie des Sciences Chimiques, 8, 591-597 (1960)
R L Crawford et al.
Journal of bacteriology, 121(3), 794-799 (1975-03-01)
Gentisate:oxygen 1,2-oxidoreductase (decyclizing) (EC 1.13.11.4; gentisate 1,2-dioxygenase) from Moraxella osloensis was purified to homogeneity as shown by polyacrylamide gel electrophoresis. The enzyme has a molecular weight of about 154,000 and gives rise to subunits of molecular weight 40,000 in the
Robin R Shields-Cutler et al.
The Journal of biological chemistry, 290(26), 15949-15960 (2015-04-12)
During Escherichia coli urinary tract infections, cells in the human urinary tract release the antimicrobial protein siderocalin (SCN; also known as lipocalin 2, neutrophil gelatinase-associated lipocalin/NGAL, or 24p3). SCN can interfere with E. coli iron acquisition by sequestering ferric iron
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service