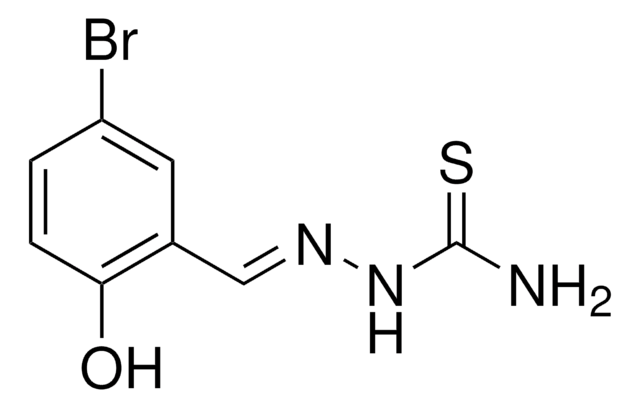
137286
5-Bromosalicylaldehyde
98%
Synonym(s):
5-Bromo-2-hydroxybenzaldehyde
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
BrC6H3-2-(OH)CHO
CAS Number:
Molecular Weight:
201.02
Beilstein:
774710
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
powder
mp
102-106 °C (lit.)
functional group
aldehyde
bromo
SMILES string
Oc1ccc(Br)cc1C=O
InChI
1S/C7H5BrO2/c8-6-1-2-7(10)5(3-6)4-9/h1-4,10H
InChI key
MKKSTJKBKNCMRV-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
5-Bromosalicylaldehyde reacts with 1,2-bis(4-chloro-2-aminophenoxy)ethane to yield Schiff base ligand 1,2-bis(2-(5-bromo-2-hydroxybenzilidenamino)-4-chlorophenoxy)ethane. It is the starting reagent for the synthesis of diarylamino-substituted N-methyl tetrahydrosalen ligand.
5-Bromosalicylaldehyde is generally used to synthesize schiff bases, and triphenyltin complexes.
5-Bromosalicylaldehyde is generally used to synthesize schiff bases, and triphenyltin complexes.
Application
5-Bromosalicylaldehyde was used for chemical derivatization during amine quantiifcation in poly (ethylene terephthalate) (PET) film and PET scaffold.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Masahito Yoshida et al.
Chemical & pharmaceutical bulletin, 68(3), 220-226 (2019-10-05)
This study demonstrates the structure-activity relationship of Col-003, a potent collagen-heat-shock protein 47 (Hsp47) interaction inhibitor. Col-003 analogues were successfully synthesized by Pd(0)-catalyzed cross-coupling reactions of 5-bromosalicylaldehyde derivatives with alkyl-metal species, and the inhibitory activities of the synthetic analogues were
Mauricio Quiroz-Guzman et al.
Dalton transactions (Cambridge, England : 2003), 40(43), 11458-11468 (2011-09-22)
A diarylamino-substituted N-methyl tetrahydrosalen (salan) ligand, (An2N)LH(2), is prepared in four steps and overall 53% yield from 5-bromosalicylaldehyde, with the key step a palladium-catalysed Hartwig-Buchwald amination of the tert-butyldimethylsilyl-protected 5-bromo-N-methylsalan ligand. Reaction of (An2N)LH(2) or its bromo analogue with Ti(O(i)Pr)(4)
Investigations on the photochromic properties of 2,6-bis(5-bromo-2-hydroxybenzylidene)cyclohexanone.
L N Corîci et al.
Photochemical & photobiological sciences : Official journal of the European Photochemistry Association and the European Society for Photobiology, 16(6), 946-953 (2017-04-27)
The network of chemical reactions of 2,6-bis(5-bromo-2-hydroxybenzylidene)cyclohexanone (BHBC) when subjected to light and different pH values has been investigated. The pH dependent species involved in the chemical network have been identified and characterized by NMR and UV-VIS spectroscopy. Direct pH
Salih Ilhan et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 118, 632-642 (2013-10-08)
A new Schiff base ligand was synthesized by reaction of 5-bromosalicylaldehyde with 1,2-bis(4-chloro-2-aminophenoxy)ethane. Then the Schiff base complexes were synthesized by the reaction of metal salts and the novel Schiff base. The molar conductivity properties of the complexes were studied
Synthesis, characterization, and cytotoxic activity of triphenyltin complexes of N-(5-bromosalicylidene)-?-amino acids
Yao Y, et al.
Main Group Metal Chemistry, 40, 93-99 (2017)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service