All Photos(1)
About This Item
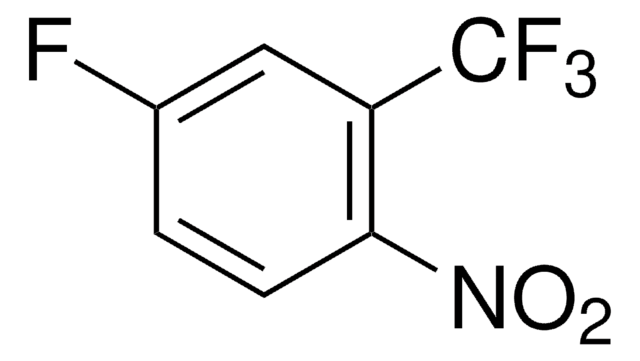
Linear Formula:
FC6H3(CN)NO2
CAS Number:
Molecular Weight:
166.11
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
mp
76-80 °C (lit.)
functional group
fluoro
nitrile
nitro
SMILES string
[O-][N+](=O)c1ccc(F)c(c1)C#N
InChI
1S/C7H3FN2O2/c8-7-2-1-6(10(11)12)3-5(7)4-9/h1-3H
InChI key
YLACBMHBZVYOAP-UHFFFAOYSA-N
General description
2-Fluoro-5-nitrobenzonitrile can be prepared from 2-fluorobenzonitrile via nitration.
Application
2-Fluoro-5-nitrobenzonitrile may be used in the preparation of:
- 2-fluoro-5-aminobenzonitrile
- ethyl 3-amino-5-nitro-benzo[b]thiophene-2-carboxylate
- 1-methyl-5-nitro-1H-indazol-3-ylamine
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Synthesis, antiproliferative activity, and in silico insights of new 3-benzoylamino-benzo [b] thiophene derivatives.
Martorana A, et al.
European Journal of Medicinal Chemistry, 90, 537-546 (2015)
Mimi L Quan et al.
Journal of medicinal chemistry, 48(6), 1729-1744 (2005-03-18)
Modification of a series of pyrazole factor Xa inhibitors to incorporate an aminobenzisoxazole as the P(1) ligand resulted in compounds with improved selectivity for factor Xa relative to trypsin and plasma kallikrein. Further optimization of the P(4) moiety led to
A method for the regioselective synthesis of 1-alkyl-1H-indazoles.
Liu HJ, et al.
Tetrahedron, 69(19), 3907-3912 (2013)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service