512346
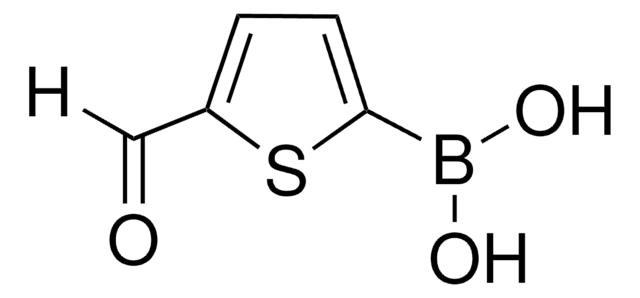
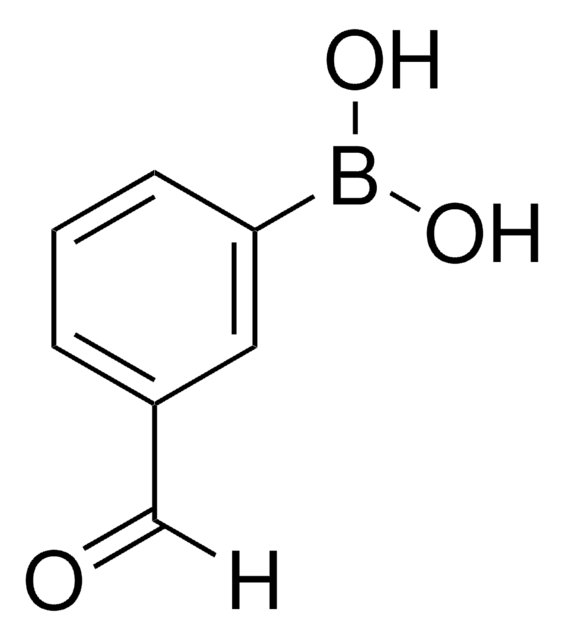
5-Formyl-2-furanylboronic acid
≥95%
Synonym(s):
5-Formyl-2-furanboronic acid
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Empirical Formula (Hill Notation):
C5H5BO4
CAS Number:
Molecular Weight:
139.90
MDL number:
UNSPSC Code:
12352103
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥95%
form
solid
mp
136 °C (dec.) (lit.)
functional group
aldehyde
SMILES string
OB(O)c1ccc(C=O)o1
InChI
1S/C5H5BO4/c7-3-4-1-2-5(10-4)6(8)9/h1-3,8-9H
InChI key
JUWYQISLQJRRNT-UHFFFAOYSA-N
Application
Reactant involved in Suzuki coupling for synthesis of stable dye-senstitized solar cells
Reactant involved in synthesis of biologically active molecules including:
Reactant involved in synthesis of biologically active molecules including:
- Heteroarylation for the synthesis of HIF-1 inhibitors
- Disalicylic acid-furanyl derivatives to inhibit ephrin binding
- HIV-1 integrase inhibitors
- Epidermal growth factor receptor inhibitors
Other Notes
Contains varying amounts of anhydride
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Qi Xia et al.
ACS applied materials & interfaces, 10(20), 17081-17088 (2018-05-03)
Effective long-term monitoring of tumor growth is significant for the evaluation of cancer therapy. Aggregation-induced emission-active near-infrared (NIR) fluorescent organic nanoparticles (TPFE-Rho dots) are designed and synthesized for long-term in vitro cell tracking and in vivo monitoring of tumor growth.
Marika Tiberi et al.
Antimicrobial agents and chemotherapy, 58(6), 3043-3052 (2014-03-13)
We report here the synthesis of 2-aminothiazolones along with their biological properties as novel anti-HIV agents. Such compounds have proven to act through the inhibition of the gp120-CD4 protein-protein interaction that occurs at the very early stage of the HIV-1
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service