441651
3-Formylphenylboronic acid
≥95%
Synonym(s):
(3-Formylbenzene)boronic acid, 3-(Dihydroxyboryl)benzaldehyde, 3-Boronobenzaldehyde, m-Formylphenylboronic acid, m-formyl-benzeneboronic acid
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
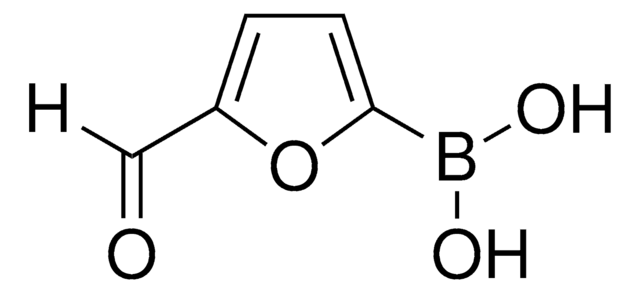
Linear Formula:
HCOC6H4B(OH)2
CAS Number:
Molecular Weight:
149.94
Beilstein:
3030769
MDL number:
UNSPSC Code:
12352103
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
≥95%
form
powder
mp
109-113 °C
functional group
aldehyde
SMILES string
OB(O)c1cccc(C=O)c1
InChI
1S/C7H7BO3/c9-5-6-2-1-3-7(4-6)8(10)11/h1-5,10-11H
InChI key
HJBGZJMKTOMQRR-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Reagent used:
Biological inhibitor of γ-glutamyltranspeptidase
Reactant involved in:
- To study the effects of boronic acid on fluoride-selective chemosignaling behavior of merocyanine dye
- As exciton-coupled CD probes for epigallocatechin gallate
Biological inhibitor of γ-glutamyltranspeptidase
Reactant involved in:
- Palladium-catalyzed homocoupling
- Suzuki coupling reactions
Other Notes
Contains varying amounts of anhydride
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Mijun Peng et al.
Journal of chromatography. A, 1474, 8-13 (2016-11-09)
Rapid and efficient extraction of bioactive glycosides from complex natural origins poses a difficult challenge, and then is often inherent bottleneck for their highly utilization. Herein, we propose a strategy to fabricate boronate affinity based surface molecularly imprinted polymers (MIPs)
Hua-Wei Liu et al.
Chemistry, an Asian journal, 12(13), 1545-1556 (2017-04-19)
Sialic acids play important roles in mammalian development, cell-cell attachment, and signaling. As cancer cells utilize their overexpressed sialylated antigens to propagate metastases, the development of probes for sialic acids is of high importance. Herein, we report three luminescent cyclometalated
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service