393290
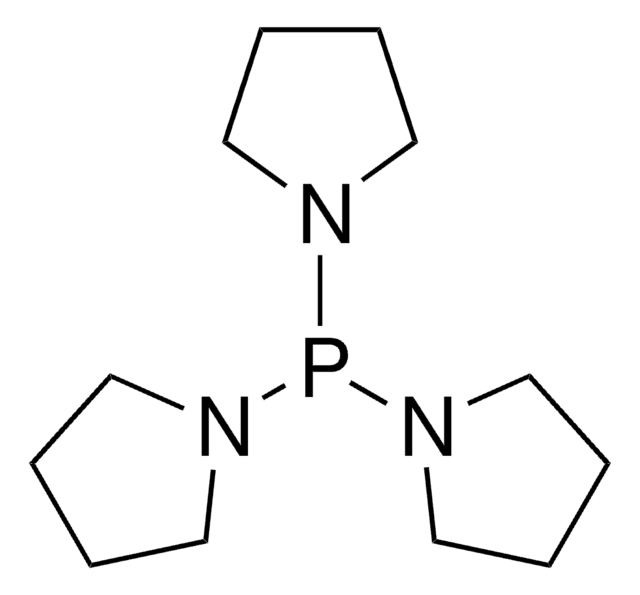
Tris(dimethylamino)phosphine
97%
Synonym(s):
(Me2N)3P, Hexamethyltriamidophosphite, Hexamethyltriaminophosphine, HMPT, Hexamethylphosphorous triamide
About This Item
Recommended Products
Quality Level
Assay
97%
form
liquid
reaction suitability
reagent type: ligand
reaction type: Reductions
reagent type: ligand
reaction type: Suzuki-Miyaura Coupling
reagent type: ligand
reaction type: Wittig Reaction
refractive index
n20/D 1.463 (lit.)
bp
48-50 °C/12 mmHg (lit.)
density
0.898 g/mL at 25 °C (lit.)
functional group
phosphine
SMILES string
CN(C)P(N(C)C)N(C)C
InChI
1S/C6H18N3P/c1-7(2)10(8(3)4)9(5)6/h1-6H3
InChI key
XVDBWWRIXBMVJV-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
- Ligand in the preparation of arene-ruthenium(II) complex which is used as a catalyst in hydration of nitriles to amides.
- Phosphorus source along with InCl3 in the synthesis of InP colloidal quantum dots (QDs).
- Deoxygenating agent for some α-dicarbonyl compounds in the presence of fullerene C60.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Carc. 1B - Eye Irrit. 2 - Flam. Liq. 3 - Muta. 1B - Skin Irrit. 2
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
80.6 °F - closed cup
Flash Point(C)
27 °C - closed cup
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Related Content
The Catalexis platform enhances catalysis by digitally optimizing catalyst selection to identify the most effective phosphine ligands for cross-coupling reactions.
The Catalexis platform enhances catalysis by digitally optimizing catalyst selection to identify the most effective phosphine ligands for cross-coupling reactions.
The Catalexis platform enhances catalysis by digitally optimizing catalyst selection to identify the most effective phosphine ligands for cross-coupling reactions.
The Catalexis platform enhances catalysis by digitally optimizing catalyst selection to identify the most effective phosphine ligands for cross-coupling reactions.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service