All Photos(1)
About This Item
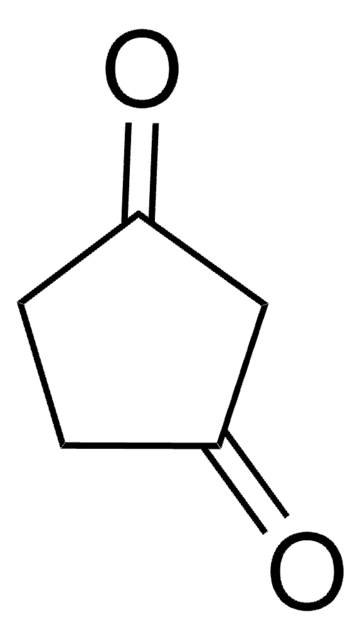
Linear Formula:
CH3OC5H5(=O)
CAS Number:
Molecular Weight:
112.13
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
99%
form
solid
mp
49-53 °C (lit.)
functional group
ether
ketone
SMILES string
COC1=CC(=O)CC1
InChI
1S/C6H8O2/c1-8-6-3-2-5(7)4-6/h4H,2-3H2,1H3
InChI key
DTWCFCILAJVNPE-UHFFFAOYSA-N
Related Categories
General description
3-Methoxy-2-cyclopenten-1-one (3-methoxycyclopent-2-enone) is a 3-methoxycycloalk-2- enone.
Application
3-Methoxy-2-cyclopenten-1-one (3-methoxycyclopent-2-enone) may be used in the following studies:
- Synthesis of 3-cyclopentyl-2-cyclopenten-1-one.
- As a starting material in the synthesis of 3-alkyl-2-aryl-2-cyclopenten-1-one oxime derivatives and kjellmanianone, an antibiotic.
- As one of the reagent in the synthesis of 3-aryl enones.
- Preparation of 1:1 diastereomeric mixture of TBS (tert-butyldimethylsilyl)-protected enones.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Tetrahedron, 47, 173-173 (1991)
Yeonjoon Kim et al.
Bioorganic & medicinal chemistry letters, 24(13), 2807-2810 (2014-05-24)
3-Alkyl-2-aryl-2-cyclopenten-1-one oxime derivatives (1) were studied as a novel class of inhibitors of tumor necrosis factor α (TNF-α) with regard to synthesis and in vitro SAR inhibition of TNF-α. The in vitro IC50 values of these compounds in rat and
Gamal A I Moustafa et al.
The Journal of organic chemistry, 77(2), 1202-1207 (2012-01-03)
The alkylation of dienolates generated from 3-methoxycycloalk-2-enones having a 3'-hydroxyl alkenyl chain provides the corresponding quaternized cycloalkenones in a highly diastereoselective manner. The high degree of stereocontrol in the α-quaternization possibly implies intervention of a rigid chelating transition state that
Discovery of orally efficacious melanin-concentrating hormone receptor-1 antagonists as antiobesity agents. Synthesis, SAR, and biological evaluation of bicyclo [3.1. 0] hexyl ureas.
McBriar MD, et al.
Journal of Medicinal Chemistry, 49(7), 1202-1207 (2012)
Patrick Y Toullec et al.
Proceedings of the National Academy of Sciences of the United States of America, 101(16), 5810-5814 (2004-04-09)
The enantioselective formation of a quaternary stereogenic center coinciding with a hydroxylation process is a very rare reaction from a homogeneous catalysis point of view. Indeed, to our knowledge, no asymmetric transition-metal-catalyzed direct hydroxylation has been reported before. We describe
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service