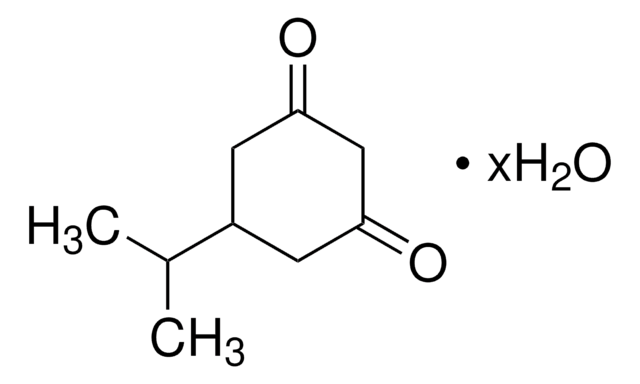
177164
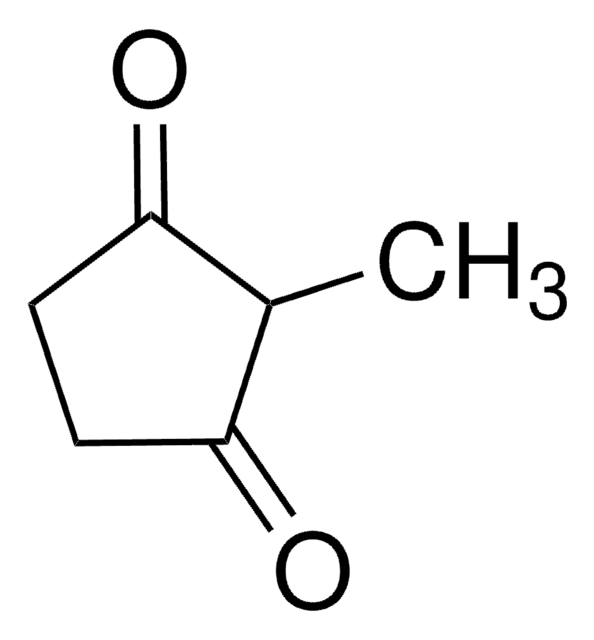
1,3-Cyclopentanedione
97%
Synonym(s):
1,3-Cyclopentadione
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
C5H6(=O)2
CAS Number:
Molecular Weight:
98.10
Beilstein:
1362728
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
form
powder
mp
149-151 °C (lit.)
functional group
ketone
storage temp.
2-8°C
SMILES string
O=C1CCC(=O)C1
InChI
1S/C5H6O2/c6-4-1-2-5(7)3-4/h1-3H2
InChI key
LOGSONSNCYTHPS-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
1,3-Cyclopentanedione was used in the synthesis of chemical probes for selective labeling of sulfenic acid proteins.
Versatile reagent for synthesis of perhydroazulenes, PGB1 analogues, and Knoevenagel products.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
The Journal of Organic Chemistry, 58, 3953-3953 (1993)
Synlett, 539-539 (1993)
Jiang Qian et al.
Chemical communications (Cambridge, England), 47(32), 9203-9205 (2011-07-09)
Facile, two-step synthesis and kinetic characterization of new chemical probes for selective labeling of sulfenic acid (-SOH) in proteins are presented. The synthesis route relies on the simple and highly efficient Michael addition of thiol containing tags or linkers to
Aldrichimica Acta, 10, 19-19 (1977)
Carole Reymond et al.
Talanta, 205, 120063-120063 (2019-08-28)
A high number of factors controlled by the experimenter has to be optimized to successfully separate, ionize and detect compounds when analyzing complex matrices by liquid chromatography hyphenated to high resolution mass spectrometry (LC-UV/MS). Key steps to manage such hyphenation
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service