All Photos(2)
About This Item
Linear Formula:
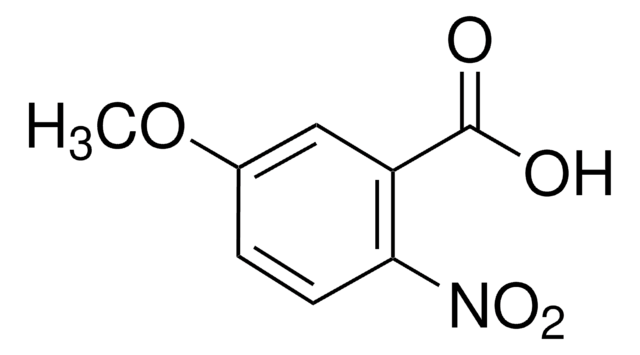
O2NC6H2(OCH3)2CO2H
CAS Number:
Molecular Weight:
227.17
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
mp
195-197 °C (lit.)
functional group
carboxylic acid
nitro
SMILES string
COc1cc(C(O)=O)c(cc1OC)[N+]([O-])=O
InChI
1S/C9H9NO6/c1-15-7-3-5(9(11)12)6(10(13)14)4-8(7)16-2/h3-4H,1-2H3,(H,11,12)
InChI key
WWCMFGBGMJAJRX-UHFFFAOYSA-N
General description
4,5-Dimethoxy-2-nitrobenzoic acid is a nitroaromatic compound.
Application
4,5-Dimethoxy-2-nitrobenzoic acid was used in the synthesis of 4,5-dimethoxy-2-nitrobenzamide and 6,7-dimethoxyquinazoline derivatives.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
R K Narla et al.
Clinical cancer research : an official journal of the American Association for Cancer Research, 4(6), 1405-1414 (1998-06-17)
The novel quinazoline derivative 4-(3'-bromo-4'-hydroxylphenyl)-amino-6,7-dimethoxyquinazoline (WHI-P154) exhibited significant cytotoxicity against U373 and U87 human glioblastoma cell lines, causing apoptotic cell death at micromolar concentrations. The in vitro antiglioblastoma activity of WHI-P154 was amplified > 200-fold and rendered selective by conjugation
J Navrátilová et al.
Folia microbiologica, 49(5), 613-615 (2005-02-11)
Two bacterial strains were isolated from forest soil by selective enrichment of the mineral medium containing 4-nitropyrocatechol as the sole carbon and energy source. Both strains could utilize 4-nitropyrocatechol and 5-nitroguaiacol. Degradation of 5-nitroguaiacol and stoichiometric release of nitrites was
P A Goodman et al.
The Journal of biological chemistry, 273(28), 17742-17748 (1998-07-04)
Exposure of B-lineage lymphoid cells to ionizing radiation induces an elevation of c-jun proto-oncogene mRNA levels. This signal is abrogated by protein-tyrosine kinase (PTK) inhibitors, indicating that activation of an as yet unidentified PTK is mandatory for radiation-induced c-jun expression.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service