178470
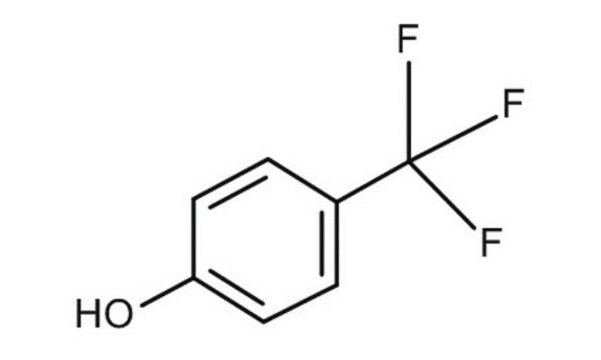
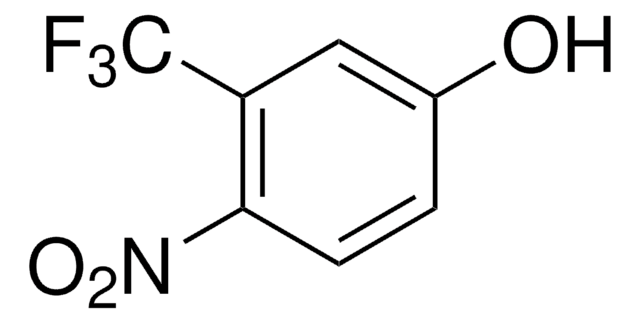
4-(Trifluoromethyl)phenol
97%
Synonym(s):
α,α,α-Trifluoro-p-cresol, 4-Hydroxybenzotrifluoride
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
CF3C6H4OH
CAS Number:
Molecular Weight:
162.11
Beilstein:
1637019
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
form
liquid
mp
45-47 °C (lit.)
functional group
fluoro
storage temp.
2-8°C
SMILES string
Oc1ccc(cc1)C(F)(F)F
InChI
1S/C7H5F3O/c8-7(9,10)5-1-3-6(11)4-2-5/h1-4,11H
InChI key
BAYGVMXZJBFEMB-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
4-(Trifluoromethyl)phenol molecule, bound at the active site of H61T (His-61→Thr) mutant, shows strong density.
4-(Trifluoromethyl)phenol also known as p-trifluoromethylphenol, is used in synthesis of polymers and monomers.
4-(Trifluoromethyl)phenol also known as p-trifluoromethylphenol, is used in synthesis of polymers and monomers.
Application
4-(Trifluoromethyl)phenol (4-hydroxybenzotrifluoride) was used in the synthesis of diaryl ether.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Oral - Eye Dam. 1 - Flam. Sol. 1 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
4.1B - Flammable solid hazardous materials
WGK
WGK 3
Flash Point(F)
183.2 °F - closed cup
Flash Point(C)
84 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
L J Urichuk et al.
Journal of chromatography. B, Biomedical sciences and applications, 698(1-2), 103-109 (1997-11-21)
An electron-capture gas chromatographic procedure was developed for the analysis of p-trifluoromethylphenol, an O-dealkylated metabolite of fluoxetine, in biological samples. A basic extraction of the biological sample was employed, followed by derivatization with pentafluorobenzenesulfonyl chloride. The internal standard, 2,4-dichlorophenol, was
Lisa Bauer et al.
ACS infectious diseases, 5(9), 1609-1623 (2019-07-16)
Enteroviruses (family Picornaviridae) comprise a large group of human pathogens against which no licensed antiviral therapy exists. Drug-repurposing screens uncovered the FDA-approved drug fluoxetine as a replication inhibitor of enterovirus B and D species. Fluoxetine likely targets the nonstructural viral
Przemysław Drzewicz et al.
Environmental technology, 40(25), 3265-3275 (2018-05-15)
A large amount of pharmaceuticals are flushed to environment via sewage system. The compounds are persistent in environment and are very difficult to remove in drinking water treatment processes. Degradation of fluoxetine (FLU) and fluvoxamine (FLX) by ferrate(VI) were investigated.
M W Fraaije et al.
The Journal of biological chemistry, 275(49), 38654-38658 (2000-09-14)
Vanillyl-alcohol oxidase (VAO) is member of a newly recognized flavoprotein family of structurally related oxidoreductases. The enzyme contains a covalently linked FAD cofactor. To study the mechanism of flavinylation we have created a design point mutation (His-61 --> Thr). In
Selina Tisler et al.
Environmental science & technology, 53(13), 7400-7409 (2019-05-29)
The present study investigates the transformation of the antidepressant fluoxetine (FLX) by photo- and biodegradation and shows similarities and differences in transformation products (TPs). TPs were identified using LC-high-resolution mass spectrometry with positive and negative electrospray ionization. In a sunlight
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service