328103
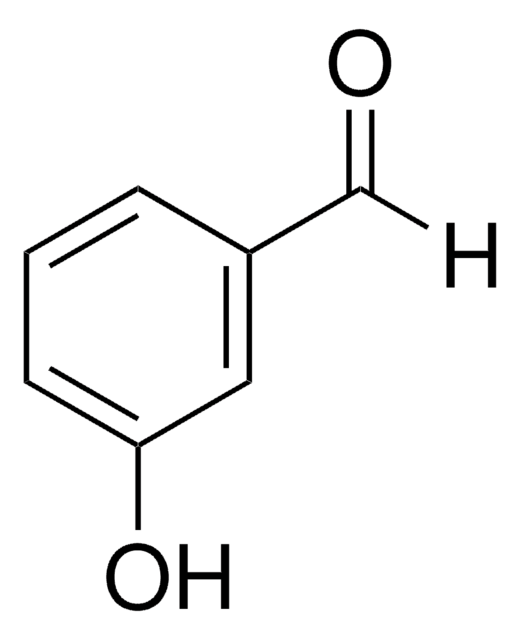
3′-Hydroxyacetophenone
≥99%
Synonym(s):
1-(3-Hydroxyphenyl)ethanone, 3-Acetylphenol, 3-Hydroxyphenylethanone, m-Hydroxyacetophenone
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
HOC6H4COCH3
CAS Number:
Molecular Weight:
136.15
Beilstein:
2040676
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥99%
bp
296 °C (lit.)
mp
90-95 °C (lit.)
density
1.1 g/mL at 25 °C (lit.)
functional group
ketone
SMILES string
CC(=O)c1cccc(O)c1
InChI
1S/C8H8O2/c1-6(9)7-3-2-4-8(10)5-7/h2-5,10H,1H3
InChI key
LUJMEECXHPYQOF-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
3′-Hydroxyacetophenone is an hydroxy-substituted alkyl phenyl ketone.
Application
3′-Hydroxyacetophenone was used in synthesis of enantiopure (-)-rivastigmine. It was also used in preparation of building block for synthesis of dendritic compounds.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
247.3 °F - closed cup
Flash Point(C)
119.6 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Synthesis of polyester dendrimers and dendrons starting from Michael reaction of acrylates with 3-hydroxyacetophenone.
Hirayama Y, et a
Tetrahedron Letters, 46(6), 1027-1030 (2005)
Kiwon Han et al.
The Journal of organic chemistry, 75(9), 3105-3108 (2010-03-30)
A practical and efficient procedure for the synthesis of rivastigmine was developed. This procedure includes dynamic kinetic resolution using a polymer-bound ruthenium complex and a lipase in combination as a key step. Enantiopure (-)-rivastigmine was obtained from commercially available 3'-hydroxyacetophenone
Katharine Moore Tibbetts et al.
The journal of physical chemistry. A, 118(37), 8170-8176 (2014-03-01)
The hydroxy-substituted alkyl phenyl ketones 2'-, 3'- and 4'- (ortho, meta, and para) hydroxyacetophenone were excited in the strong-field regime with wavelengths ranging from 1200-1500 nm to produce the respective radical cations. For 2'- and 3'-hydroxyacetophenone, the parent molecular ion
Takashi Otani et al.
Bioorganic & medicinal chemistry letters, 18(12), 3582-3584 (2008-05-31)
m-Acetylphenyl-beta-d-glucopyranosides and m-acetylphenyl-alpha/beta-d-mannopyranosides were synthesized by the Koenigs-Knorr, Mitsunobu, and Helferich reactions as key glycosylation reactions, respectively. Their spectroscopic properties and antioxidative activities were characterized as potential ultraviolet B-ray absorbents.
Stefania Ferrari et al.
Journal of medicinal chemistry, 54(1), 211-221 (2010-12-04)
Folate analogue inhibitors of Leishmania major pteridine reductase (PTR1) are potential antiparasitic drug candidates for combined therapy with dihydrofolate reductase (DHFR) inhibitors. To identify new molecules with specificity for PTR1, we carried out a virtual screening of the Available Chemicals
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service