138827
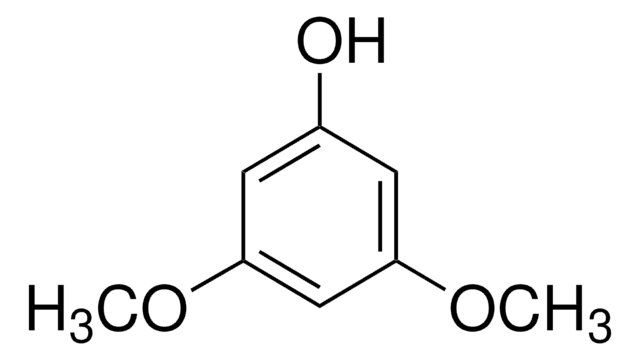
1,3,5-Trimethoxybenzene
ReagentPlus®, ≥99%
Synonym(s):
Phloroglucinol trimethyl ether
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
C6H3(OCH3)3
CAS Number:
Molecular Weight:
168.19
Beilstein:
1307993
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
product line
ReagentPlus®
Assay
≥99%
form
solid
bp
255 °C (lit.)
mp
50-53 °C (lit.)
SMILES string
COc1cc(OC)cc(OC)c1
InChI
1S/C9H12O3/c1-10-7-4-8(11-2)6-9(5-7)12-3/h4-6H,1-3H3
InChI key
LKUDPHPHKOZXCD-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
1,3,5-Trimethoxybenzene effectively cleaves p-methoxybenzyl protecting group on various alcohols and acids. It is the major scent compound present in Chinese rose species.
Application
1,3,5-Trimethoxybenzene was used to study the photodeoxygenation of 1,2-benzodiphenylene sulfoxide. It was employed as secondary standard in quantitative proton NMR spectroscopy of pharmaceuticals.
Legal Information
ReagentPlus is a registered trademark of Merck KGaA, Darmstadt, Germany
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Nicolas Kern et al.
The Journal of organic chemistry, 77(20), 9227-9235 (2012-09-26)
The p-methoxybenzyl protecting group (PMB) on various alcohols and an acid was efficiently and selectively cleaved by the action of a catalytic amount of silver(I) hexafluoroantimonate combined with 0.5 equiv of 1,3,5-trimethoxybenzene in dichloromethane at 40 °C.
Xichen Cai et al.
The journal of physical chemistry. A, 111(22), 4743-4747 (2007-05-08)
Bimolecular hole transfer quenching of the 1,3,5-trimethoxybenzene radical cation (TMB*+) in the excited state (TMB*+*) by hole quenchers (Q) such as biphenyl (Bp), naphthalene (Np), anisole (An), and benzene (Bz) with higher oxidation potentials than that of TMB was directly
Xichen Cai et al.
The journal of physical chemistry. A, 111(10), 1788-1791 (2007-02-14)
One-electron oxidation of alcohols such as methanol, ethanol, and 2-propanol by 1,3,5-trimethoxybenzene radical cation (TMB*+) in the excited state (TMB*+*) was observed during the two-color two-laser flash photolysis. TMB*+ was formed by the photoinduced bimolecular electron-transfer reaction from TMB to
Tao Fang et al.
Journal of the American Chemical Society, 134(17), 7545-7552 (2012-04-06)
The development of selectively protected monosaccharide building blocks that can reliably be glycosylated with a wide variety of acceptors is expected to make oligosaccharide synthesis a more routine operation. In particular, there is an urgent need for the development of
O Chassany et al.
Alimentary pharmacology & therapeutics, 25(9), 1115-1123 (2007-04-19)
Abdominal pain is the predominant symptom in irritable bowel syndrome patients. Phloroglucinol and its methylated derivative are antispasmodic agents acting on smooth muscle. To evaluate the efficacy of phloroglucinol/trimethylphloroglucinol on pain intensity during an acute exacerbation of pain of irritable
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service