304484
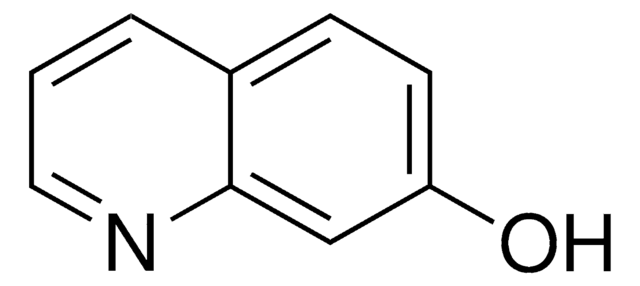
6-Hydroxyquinoline
95%
Synonym(s):
6-Quinolinol, 6-Hydroxyquinoline
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Empirical Formula (Hill Notation):
C9H7NO
CAS Number:
Molecular Weight:
145.16
Beilstein:
113196
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
95%
mp
188-190 °C (lit.)
SMILES string
Oc1ccc2ncccc2c1
InChI
1S/C9H7NO/c11-8-3-4-9-7(6-8)2-1-5-10-9/h1-6,11H
InChI key
OVYWMEWYEJLIER-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
6-Hydroxyquinoline is an ideal photoacid system for exploring excited-state proton transfer (ESPT) reactions. The excited-state proton transfer and geminate recombination of 6-hydroxyquinoline encaged in catalytic Na+-exchanged faujasite zeolites X and Y have been explored by measuring steady-state and picosecond time-resolved spectra.
Application
6-Hydroxyquinoline was used in synthesis of 2,6-substituted-benzo[d]thiazole analogs and 2,4-substituted-benzo[d]thiazole analogs.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Yu-Hui Liu et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 128, 280-284 (2014-04-01)
6-Hydroxyquinoline (6HQ) is an ideal photoacid system for exploring excited-state proton transfer (ESPT) reactions. We have previously (Mahata et al. (2002)) shown that the ESPT reaction between 6HQ and trimethylamine (TMA) leads to an "unusual" emission in the 440-450 nm
Katharigatta Narayanaswamy Venugopala et al.
European journal of medicinal chemistry, 65, 295-303 (2013-06-04)
A novel and efficient one pot synthesis was developed for 2,6-substituted-benzo[d]thiazole analogues 4a-k and 2,4-substituted-benzo[d]thiazole analogues 4l-pvia three component condensation reaction of substituted arylaldehyde, 2-amino-6-halo/4-methyl-benzo[d]thiazole and 2-naphthol or 6-hydroxyquinoline in presence of 10% w/v NaCl in water by microwave method.
G Bott et al.
Biological chemistry Hoppe-Seyler, 372(6), 381-383 (1991-06-01)
Two strains, using 6-hydroxyquinoline as sole source of energy, carbon and nitrogen, have been isolated. These bacteria, designated 31/1 Fa1 and 31/2 A1, are also able to degrade quinoline. According to their physiological properties strain 31/1 Fa1 has been identified
Selective phenolic acylation of 10-hydroxycamptothecin using poly (ethylene glycol) carboxylic acid.
Richard B Greenwald et al.
Bioorganic & medicinal chemistry letters, 13(3), 577-580 (2003-02-05)
Selective acylation of the phenolic hydroxyl group of 10-hydroxycamptothecin has been accomplished using phenyl dichlorophosphate. Additional modification of the 10-OH as an ether permits a 20-acyl derivative to be synthesized. This result along with data from a 6-hydroxyquinoline model strongly
Sun-Young Park et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 16(42), 12609-12615 (2010-09-21)
The excited-state proton transfer and geminate recombination of 6-hydroxyquinoline (6HQ) encaged in catalytic Na(+)-exchanged faujasite zeolites X (NaX) and Y (NaY) have been explored by measuring steady-state and picosecond time-resolved spectra. The pathways and rate constants of proton transfer of
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service