270873
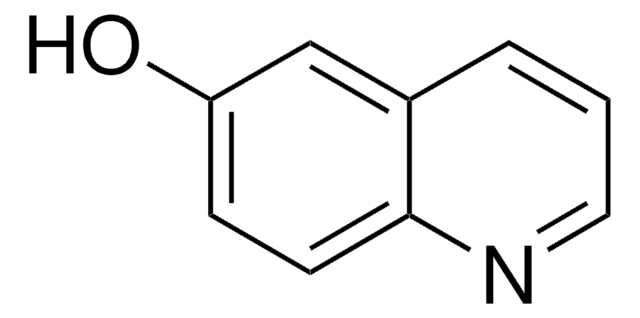
2-Hydroxyquinoline
98%
Synonym(s):
2-Quinolinol, Carbostyril
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C9H7NO
CAS Number:
Molecular Weight:
145.16
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
crystals
mp
198-199 °C (lit.)
solubility
alcohol: soluble(lit.)
diethyl ether: soluble(lit.)
dilute HCl: soluble(lit.)
water: slightly soluble (1 g/950mL)(lit.)
SMILES string
Oc1ccc2ccccc2n1
InChI
1S/C9H7NO/c11-9-6-5-7-3-1-2-4-8(7)10-9/h1-6H,(H,10,11)
InChI key
LISFMEBWQUVKPJ-UHFFFAOYSA-N
General description
2-Hydroxyquinoline is a specific inhibitor of plaque paraoxonase1 (PON1).
Application
2-Hydroxyquinoline was used to obtain the design of the platinum(IV) complexes with intense bands shifted towards longer wavelengths. The Pt(IV) complexes are inert stable prodrugs that were photoactivated to produce Pt(II) species with promising anticancer activity.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Dam. 1 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Shirin Biglari et al.
Microbial drug resistance (Larchmont, N.Y.), 23(5), 545-555 (2016-11-18)
Multidrug-resistant (MDR) Acinetobacter baumannii has increasingly emerged as an important nosocomial pathogen. The aim of this study was to determine the resistance profiles and genetic diversity in A. baumannii clinical isolates in a tertiary medical center in Malaysia. The minimum
Antonios Keirouz et al.
Journal of nanobiotechnology, 18(1), 51-51 (2020-03-20)
The state-of-the-art hernia meshes, used in hospitals for hernia repair, are predominantly polymeric textile-based constructs that present high mechanical strength, but lack antimicrobial properties. Consequently, preventing bacterial colonization of implanted prosthetic meshes is of major clinical relevance for patients undergoing
Alexander J Bones et al.
Experimental parasitology, 196, 28-37 (2018-12-07)
Cryptosporidium is a genus of single celled parasites capable of infecting a wide range of animals including humans. Cryptosporidium species are members of the phylum apicomplexa, which includes well-known genera such as Plasmodium and Toxoplasma. Cryptosporidium parasites cause a severe
Kamya Bhatnagar et al.
PloS one, 14(11), e0224650-e0224650 (2019-11-07)
The evolution of antibiotic resistance is influenced by a variety of factors, including the availability of resistance mutations, and the pleiotropic effects of such mutations. Here, we isolate and characterize chromosomal quinolone resistance mutations in E. coli, in order to
Edyta Stec et al.
Chembiochem : a European journal of chemical biology, 12(11), 1724-1730 (2011-06-15)
Aurachins are quinoline alkaloids isolated from the myxobacterium Stigmatella aurantiaca. They are substituted with an isoprenoid side chain and act as potent inhibitors in the electron transport chain. A biosynthetic gene cluster that contains at least five genes (auaA-auaE) has
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service