268402
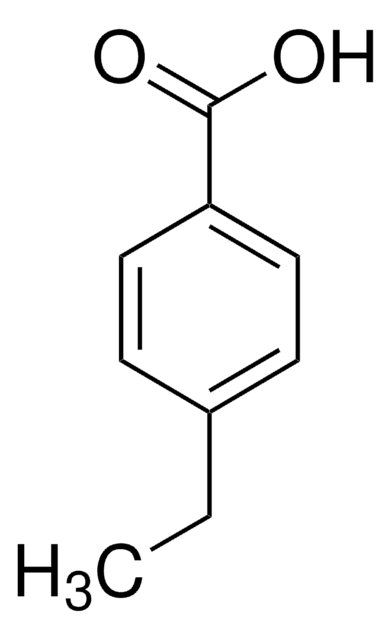
4-Isopropylbenzoic acid
≥96%
Synonym(s):
Cumic acid, Cuminic acid
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
(CH3)2CHC6H4CO2H
CAS Number:
Molecular Weight:
164.20
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥96%
mp
117-120 °C (lit.)
solubility
alcohol: soluble(lit.)
diethyl ether: soluble(lit.)
sulfuric acid: soluble(lit.)
water: slightly soluble(lit.)
functional group
carboxylic acid
SMILES string
CC(C)c1ccc(cc1)C(O)=O
InChI
1S/C10H12O2/c1-7(2)8-3-5-9(6-4-8)10(11)12/h3-7H,1-2H3,(H,11,12)
InChI key
CKMXAIVXVKGGFM-UHFFFAOYSA-N
Related Categories
Application
4-Isopropylbenzoic acid (cuminic acid) was used in the synthesis of three new triorganotin carboxylates bearing methyl, butyl and phenyl substituents at tin, respectively. The products were fully characterized by spectroscopic and thermal techniques, with regard to the coordination number of tin atom, in solution and in the solid state.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
R W Eaton
Journal of bacteriology, 178(5), 1351-1362 (1996-03-01)
Pseudomonas putida F1 utilizes p-cumate (p-isopropylbenzoate) as a growth substrate by means of an eight-step catabolic pathway. A 35.75-kb DNA segment, within which the cmt operon encoding the catabolism of p-cumate is located, was cloned as four separate overlapping restriction
Angiolini L, et al.
Journal of Organometallic Chemistry, 691(9), 1965-1972 (2006)
Bruno Gaillet et al.
Biotechnology and bioengineering, 106(2), 203-215 (2010-02-24)
Fast and efficient production of recombinant proteins for structural and functional studies is a crucial issue for research and for industry. To this end, we have developed an efficient system to generate in less than 2 months, starting from the
R W Eaton
Journal of bacteriology, 179(10), 3171-3180 (1997-05-01)
Pseudomonas putida F1 utilizes p-cymene (p-isopropyltoluene) by an 11-step pathway through p-cumate (p-isopropylbenzoate) to isobutyrate, pyruvate, and acetyl coenzyme A. The cym operon, encoding the conversion of p-cymene to p-cumate, is located just upstream of the cmt operon, which encodes
Bruno Gaillet et al.
Biotechnology progress, 23(1), 200-209 (2007-02-03)
To facilitate and accelerate the production of eukaryotic proteins with correct post-translational modifications, we have developed a protein production system based on the transduction of Chinese hamster ovary (CHO) cells using adenovirus vectors (AdVs). We have engineered a CHO cell
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service