250899
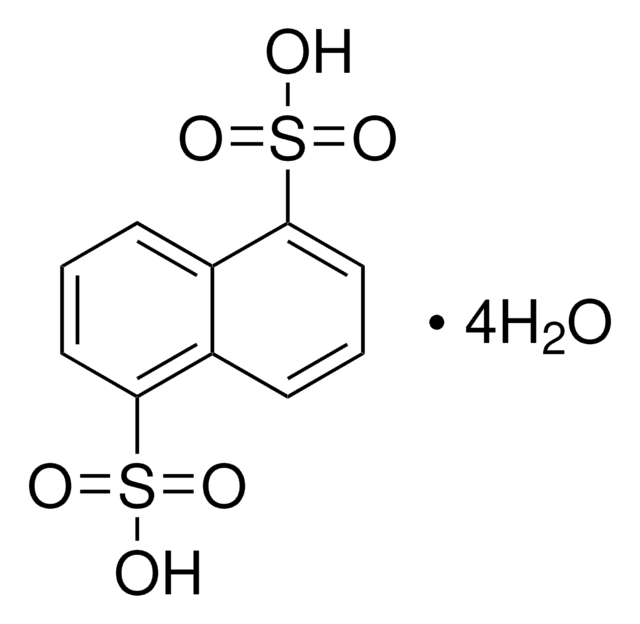
1,5-Naphthalenedisulfonic acid disodium salt hydrate
95%
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
C10H6(SO3Na)2 · xH2O
CAS Number:
Molecular Weight:
332.26 (anhydrous basis)
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
95%
form
powder
functional group
sulfonic acid
SMILES string
[Na+].[Na+].[O-]S(=O)(=O)c1cccc2c(cccc12)S([O-])(=O)=O
InChI
1S/C10H8O6S2.2Na/c11-17(12,13)9-5-1-3-7-8(9)4-2-6-10(7)18(14,15)16;;/h1-6H,(H,11,12,13)(H,14,15,16);;/q;2*+1/p-2
InChI key
YGSZNSDQUQYJCY-UHFFFAOYSA-L
General description
The liposomal entrapment of 1,5-naphthalenedisulfonic acid disodium salt in phospholipid vesicles has been studied. Doubly substituted aromatic dianions of 1,5-naphthalenedisulfonic acid disodium salt has been investigated by collision-induced dissociation, along with infrared multiple photon dissociation/detachment techniques.
Other Notes
Contains sodium chloride
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
H C Chang et al.
Journal of pharmaceutical sciences, 83(7), 1043-1046 (1994-07-01)
The liposomal entrapment of suramin and similar compounds in phospholipid vesicles was examined. For dipalmitoylphosphatidylcholine (DPPC) liposomes, entrapment percentages ranged from 25 to 65% with 3-25 mM phospholipid for aqueous solutions containing 0.07 mM of suramin. Incorporation of 30-50 mol
Shaun Ard et al.
The Journal of chemical physics, 132(9), 094301-094301 (2010-03-10)
Collision-induced dissociation (CID), along with infrared multiple photon dissociation/detachment (IRMPD) techniques, is utilized to study a series of doubly substituted aromatic dianions containing sulfonate and carboxylate functionalities (1,2- and 1,3-benzenedisulfonate, 1,5-naphthalenedisulfonate, 2,6-naphthalenedisulfonate, 4-sulfobenzoate, 2,6-naphthalenedicarboxylate, and terephthalate dianions). The molecules were
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service