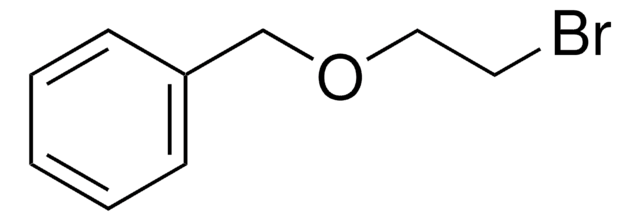
245631
Benzyl bromoacetate
96%
Synonym(s):
Bromoacetic acid benzyl ester
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
BrCH2COOCH2C6H5
CAS Number:
Molecular Weight:
229.07
Beilstein:
973658
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
96%
form
liquid
refractive index
n20/D 1.544 (lit.)
bp
166-170 °C/22 mmHg (lit.)
density
1.446 g/mL at 25 °C (lit.)
functional group
bromo
SMILES string
BrCC(=O)OCc1ccccc1
InChI
1S/C9H9BrO2/c10-6-9(11)12-7-8-4-2-1-3-5-8/h1-5H,6-7H2
InChI key
JHVLLYQQQYIWKX-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Benzyl bromoacetate was used in the alkylation of (-)-2,3-O-isopropylidene-D-threitol that afforded lipopeptide, 2-[(4R,5R)-5-({[(9H-fluoren-9-yl)methoxy]carbonylaminomethyl}-2,2-dimethyl-1,3-dioxolan-4-yl)methoxy]acetic acid.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
235.4 °F - closed cup
Flash Point(C)
113 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Toru Masaka et al.
Chemical & pharmaceutical bulletin, 61(11), 1184-1187 (2013-08-28)
A new component for the solid phase peptide synthesis of lipopeptide, 2-[(4R,5R)-5-({[(9H-fluoren-9-yl)methoxy]carbonylaminomethyl}-2,2-dimethyl-1,3-dioxolan-4-yl)methoxy]acetic acid (2), was designed and synthesized from (-)-2,3-O-isopropylidene-D-threitol (3) in 4 steps. The key step was the selective alkylation of 3 with benzyl bromoacetate in the presence of
Michael Kahnt et al.
Molecules (Basel, Switzerland), 24(12) (2019-06-20)
In this study, we report the synthesis of several amine-spacered conjugates of ursolic acid (UA) and 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA). Thus, a total of 11 UA-DOTA conjugates were prepared holding various oligo-methylene diamine spacers as well as different substituents at the
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service
![1,8-Diazabicyclo[5.4.0]undec-7-ene 98%](/deepweb/assets/sigmaaldrich/product/structures/120/564/5b373e23-1624-489c-8efb-692de0f96ffb/640/5b373e23-1624-489c-8efb-692de0f96ffb.png)