135615
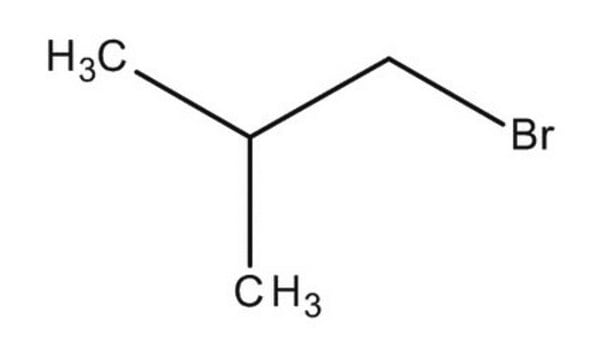
2-Bromo-2-methylpropane
98%
Synonym(s):
2-methyl-2-bromopropane, tert-Butyl bromide
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
(CH3)3CBr
CAS Number:
Molecular Weight:
137.02
Beilstein:
1730892
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
liquid
contains
0.5% potassium carbonate as stabilizer
refractive index
n20/D 1.4279 (lit.)
bp
71-73 °C (lit.)
mp
−20 °C (lit.)
solubility
H2O: insoluble
organic solvents: miscible
density
1.22 g/mL at 20 °C (lit.)
functional group
alkyl halide
bromo
SMILES string
CC(C)(C)Br
InChI
1S/C4H9Br/c1-4(2,3)5/h1-3H3
InChI key
RKSOPLXZQNSWAS-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
2-Bromo-2-methylpropane (tert-Butyl bromide) is a versatile reactant in organic synthesis, facilitating the introduction of the tert-butyl group. It causes the massive deguanylation of guanine based-nucleosides and massive deadenylation of adenine based-nucleosides.
Application
2-Bromo-2-methylpropane was used to study the massive deadenylation of adenine based-nucleosides induced by halogenated alkanes under physiological conditions.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Flam. Liq. 2
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
60.8 °F - closed cup
Flash Point(C)
16 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Deguanylation of guanine based-nucleosides and calf thymus DNA induced by halogenated alkanes at the physiological condition.
Sherchan J and Lee E-S.
Bull. Korean Chem. Soc., 30(12), 2318-2328 (2009)
Deadenylation of Adenine Based-Nucleosides and Calf thymus DNA Induced by Halogenated Alkanes at the Physiological Condition.
Sherchan J, et al.
Bull. Korean Chem. Soc., 30(10), 2318-2328 (2009)
tert-Butyl Bromide-Promoted Intramolecular Cyclization of 2-Arylamino Phenyl Ketones and Its Combination with Cu-Catalyzed C-N Coupling: Synthesis of Acridines at Room Temperature
Z Cao, et al.
The Journal of Organic Chemistry, 85, 10167-10174 (2020)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service