All Photos(1)
About This Item
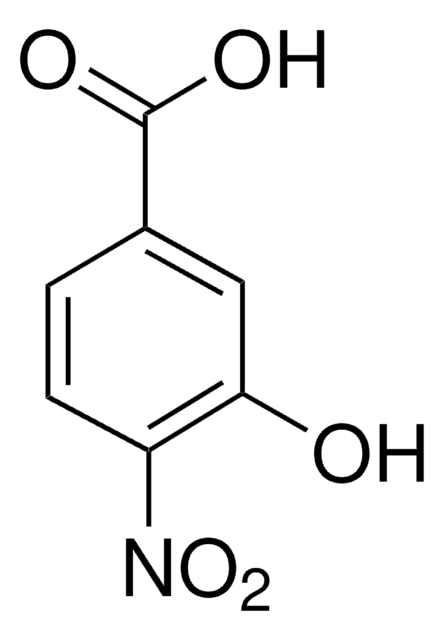
Linear Formula:
HOC6H3(NO2)CO2H
CAS Number:
Molecular Weight:
183.12
Beilstein:
2213134
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
form
solid
mp
183-186 °C (lit.)
functional group
carboxylic acid
nitro
SMILES string
OC(=O)c1ccc(O)c(c1)[N+]([O-])=O
InChI
1S/C7H5NO5/c9-6-2-1-4(7(10)11)3-5(6)8(12)13/h1-3,9H,(H,10,11)
InChI key
QRYSWXFQLFLJTC-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
4-Hydroxy-3-nitrobenzoic acid was coupled to ionic liquid-immobilized o-phenylenediamine to form benzimidazole derivatives.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Kaushik Chanda et al.
ACS combinatorial science, 14(2), 115-123 (2012-01-24)
A novel and efficient diversity-oriented synthetic approach was employed to access the benzo[d]oxazol-5-yl-1H-benzo[d]imidazole on ionic liquid support, which helps to absorb microwave irradiation. In this paper, we successfully coupled 4-hydroxy-3-nitrobenzoic acid onto ionic liquid-immobilized o-phenylenediamine, which subsequently underwent an acid
Yuefei Ji et al.
Water research, 123, 249-257 (2017-07-04)
As promising in-situ chemical oxidation (ISCO) technologies, sulfate radical-based advanced oxidation processes (SR-AOPs) are applied in wastewater treatment and groundwater remediation in recent years. In this contribution, we report for the first time that, thermally activated persulfate oxidation of phenol
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service