All Photos(1)
About This Item
Linear Formula:
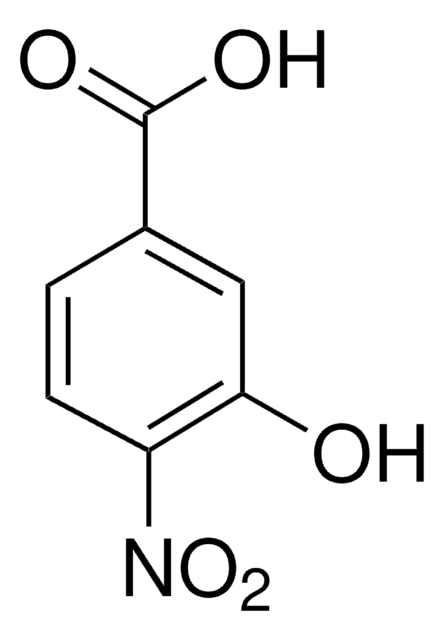
HOC6H3(NO2)CO2H
CAS Number:
Molecular Weight:
183.12
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
mp
235-239 °C (lit.)
functional group
carboxylic acid
nitro
SMILES string
OC(=O)c1ccc(cc1O)[N+]([O-])=O
InChI
1S/C7H5NO5/c9-6-3-4(8(12)13)1-2-5(6)7(10)11/h1-3,9H,(H,10,11)
InChI key
UKWUOTZGXIZAJC-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
2-Hydroxy-4-nitrobenzoic acid is metabolized to 2,4-dihydroxybenzoic acid (2,4-DHBA) by a mono-oxygenase with the concomitant release of chloride and nitrite ions.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Novel pathway for the degradation of 2-chloro-4-nitrobenzoic acid by Acinetobacter sp. strain RKJ12.
Dhan Prakash et al.
Applied and environmental microbiology, 77(18), 6606-6613 (2011-08-02)
The organism Acinetobacter sp. RKJ12 is capable of utilizing 2-chloro-4-nitrobenzoic acid (2C4NBA) as a sole source of carbon, nitrogen, and energy. In the degradation of 2C4NBA by strain RKJ12, various metabolites were isolated and identified by a combination of chromatographic
Behnoush Hajian et al.
Cell chemical biology, 26(6), 781-791 (2019-04-02)
The folate biosynthetic pathway offers many druggable targets that have yet to be exploited in tuberculosis therapy. Herein, we have identified a series of small molecules that interrupt Mycobacterium tuberculosis (Mtb) folate metabolism by dual targeting of dihydrofolate reductase (DHFR), a
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service