17356
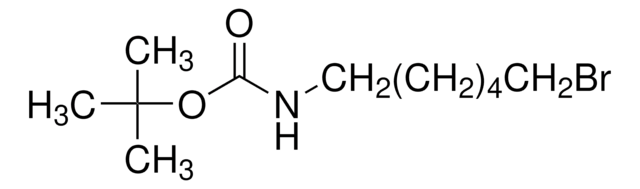
3-(Boc-amino)propyl bromide
≥96.0% (GC)
Synonym(s):
tert-Butyl N-(3-bromopropyl)carbamate
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
Br(CH2)3NHCO2C(CH3)3
CAS Number:
Molecular Weight:
238.12
Beilstein:
4176344
MDL number:
UNSPSC Code:
12352200
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥96.0% (GC)
reaction suitability
reagent type: cross-linking reagent
mp
37-39 °C
functional group
Boc
amine
bromo
storage temp.
2-8°C
SMILES string
BrCCCNC(OC(C)(C)C)=O
InChI
1S/C8H16BrNO2/c1-8(2,3)12-7(11)10-6-4-5-9/h4-6H2,1-3H3,(H,10,11)
InChI key
IOKGWQZQCNXXLD-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
3-(Boc-amino)propyl bromide can be used as an alkylating reagent for the synthesis of:
It can also be used for the post-polymerization quaternization of polymers to synthesize functional cationic polymers and antimicrobial agents.
- Benzydamine analogs to be used as activators for soluble guanylate cyclase.
- N-substituted chromenotriazolopyrimidine, human murine double minute 2 (MDM2) inhibitor.
- Protected amines from piperidine derivatives to be further used for synthesis of sulfonamide series.
It can also be used for the post-polymerization quaternization of polymers to synthesize functional cationic polymers and antimicrobial agents.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Improvement of the synthesis and pharmacokinetic properties of chromenotriazolopyrimidine MDM2-p53 protein-protein inhibitors.
Beck HP, et al.
Bioorganic & Medicinal Chemistry Letters, 21(9), 2752-2755 (2011)
Identification of selective 8-(piperidin-4-yloxy) quinoline sulfone and sulfonamide histamine H1 receptor antagonists for use in allergic rhinitis.
Procopiou PA, et al.
Bioorganic & Medicinal Chemistry Letters, 27(21), 4914-4919 (2017)
Synthesis and biological evaluation of novel pyrazoles and indazoles as activators of the nitric oxide receptor, soluble guanylate cyclase.
Selwood DL, et al.
Journal of medicinal chemistry, 44(1), 78-93 (2001)
Design, syntheses and evaluation of hemocompatible pegylated-antimicrobial polymers with well-controlled molecular structures.
Venkataraman S, et al.
Biomaterials, 31(7), 1751-1756 (2010)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service