762016
3-Azido-1-propanamine
≥95%
Synonym(s):
3-Azidopropylamine
About This Item
Recommended Products
Quality Level
Assay
≥95%
form
liquid
reaction suitability
reaction type: click chemistry
refractive index
n20/D 1.471
density
1.020 g/mL at 25 °C
storage temp.
−20°C
SMILES string
NCCCN=[N+]=[N-]
InChI
1S/C3H8N4/c4-2-1-3-6-7-5/h1-4H2
InChI key
OYBOVXXFJYJYPC-UHFFFAOYSA-N
General description
- Bismethylolpropionic acid (bis-MPA) monomers with azide functional group to generate high-generation dendrimers.,
- Clickable zinc tetraphenylporphyrin scaffold with an azido group through click chemistry applicable in photodynamic therapy.
Application
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Oral - Flam. Liq. 3
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
140.0 °F
Flash Point(C)
60 °C
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Drug discovery process by utilizing chemistry reaction of Cu(I)-catalyzed Huisgen 1,3-dipolar cycloaddition of terminal alkynes with organoazides to yield 1,4-disubstituted 1,2,3-triazoles.
Drug discovery process by utilizing chemistry reaction of Cu(I)-catalyzed Huisgen 1,3-dipolar cycloaddition of terminal alkynes with organoazides to yield 1,4-disubstituted 1,2,3-triazoles.
Drug discovery process by utilizing chemistry reaction of Cu(I)-catalyzed Huisgen 1,3-dipolar cycloaddition of terminal alkynes with organoazides to yield 1,4-disubstituted 1,2,3-triazoles.
Drug discovery process by utilizing chemistry reaction of Cu(I)-catalyzed Huisgen 1,3-dipolar cycloaddition of terminal alkynes with organoazides to yield 1,4-disubstituted 1,2,3-triazoles.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service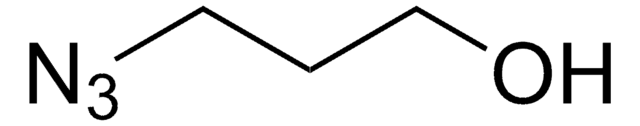


![2-[2-(2-Azidoethoxy)ethoxy]ethanol solution ~0.5 M in tert-butyl methyl ether](/deepweb/assets/sigmaaldrich/product/structures/374/007/eea7ca74-41e4-4aac-af71-c93c37ec0a5a/640/eea7ca74-41e4-4aac-af71-c93c37ec0a5a.png)