PC116
Anti-ATM (Ab-3) (819-844) Rabbit pAb
liquid, Calbiochem®
Synonym(s):
Anti-Ataxia Telangiectasia
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
UNSPSC Code:
12352203
NACRES:
NA.43
Recommended Products
biological source
rabbit
Quality Level
antibody form
purified antibody
antibody product type
primary antibodies
clone
polyclonal
form
liquid
contains
≤0.1% sodium azide as preservative
species reactivity
human
manufacturer/tradename
Calbiochem®
storage condition
do not freeze
isotype
IgG
shipped in
wet ice
storage temp.
2-8°C
target post-translational modification
unmodified
Gene Information
human ... ATM(472)
General description
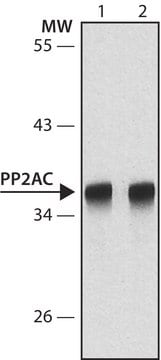
Anti-ATM (Ab-3) (819-844), rabbit polyclonal, recognizes the ~350 kDa ATM protein in Daudi and HeLa cells. It is validated for Western blotting and immunoprecipitation.
Purified rabbit polyclonal antibody. Recognizes the ~350 kDa ATM protein.
Recognizes the ~350 kDa ATM protein in Daudi and HeLa cells.
Immunogen
Human
a synthetic peptide (CKSLASFIKKPFDRGEVESMEDDTNG) corresponding to amino acids 819-844 of human ATM
Application
Immunoblotting (2 µg/ml, see comments)
Immunoprecipitation (2 µg)
Immunoprecipitation (2 µg)
Packaging
Please refer to vial label for lot-specific concentration.
Warning
Toxicity: Standard Handling (A)
Physical form
In 50 mM sodium phosphate buffer, 0.2% gelatin.
Analysis Note
Negative Control
GM02052A cells
GM02052A cells
Positive Control
Daudi or HeLa cells
Daudi or HeLa cells
Other Notes
For immunoblotting use 100-150 µg cell lysate on a 5% acrylamide gel (SDS/PAGE) and transferred to nitrocellulose using semi-dry transfer at 9V constant voltage for 2 h. Detection of antibody/antigen complexes is done using HRP conjugated goat anti-rabbit IgG at 25 ng/ml (Cat. No. DC03L).
1. Anti-ATM (Ab-3) Rabbit pAb immunoblots and immunoprecipitates a 350 kDa protein in lysates of normal and transformed cells or tumor lines derived from individuals homozygous wild type for the ATM gene; whereas in cells derived from patients with Ataxia-Telangiectasia and carrying homozygous inactivating mutations in ATM, no 350 kDa protein is detected.
2. Anti-ATM (Ab-3) Rabbit pAb may non-specifically detect smaller molecular weight proteins present in both ATM mutant and wild type cells. Careful titering of primary and secondary antibodies is recommended.
3. Immunoblotting of p350ATM requires loading 100-150 µg cell lysate on low percentage acrylamide gels (5%) (SDS/PAGE) with electrophoresis performed until the 200 kDa molecular weight marker has migrated halfway through the gel. Semi-dry electrophoretic transfer is for 2 h at 9V constant voltage. Tank transfer is overnight at 40 V constant voltage.
4. To transfer the gel for blotting, lay a dry piece of Whatman 3MM Chromatography paper over the wet gel. Carefully peel the 3MM paper and gel off the glass plate and immerse, gel side up, in transfer buffer until 3MM paper is thoroughly wet. Remove bubbles by rolling a pipette across the surface of the gel.
5. To confirm detection of p350ATM, a cell line carrying a truncating mutation in ATM should be used as a negative control. Cell lines from A-T patients which show no detectable band at 350 kDa, can be obtained from the National Institute of General Medical Sciences Human Genetic Mutant Cell Repository at the Coriell Institute for Medical Research, 401 Haddon Avenue, Camden, NJ 08103.
6. For immunoprecipitations, prepare nuclear lysates as described. Immunoprecipitate p350ATM using 2 µg of Anti-ATM (Ab-3) Rabbit pAb and Protein A-Agarose (Cat. No. IP06). Detection can be after metabolic labeling with 35S methionine followed by autoradiography, or alternatively, immunoprecipitated proteins can be displayed on 5% SDS/PAGE, transferred to nitrocellulose and then blotted as above using Anti-ATM (Ab-3).
1. Anti-ATM (Ab-3) Rabbit pAb immunoblots and immunoprecipitates a 350 kDa protein in lysates of normal and transformed cells or tumor lines derived from individuals homozygous wild type for the ATM gene; whereas in cells derived from patients with Ataxia-Telangiectasia and carrying homozygous inactivating mutations in ATM, no 350 kDa protein is detected.
2. Anti-ATM (Ab-3) Rabbit pAb may non-specifically detect smaller molecular weight proteins present in both ATM mutant and wild type cells. Careful titering of primary and secondary antibodies is recommended.
3. Immunoblotting of p350ATM requires loading 100-150 µg cell lysate on low percentage acrylamide gels (5%) (SDS/PAGE) with electrophoresis performed until the 200 kDa molecular weight marker has migrated halfway through the gel. Semi-dry electrophoretic transfer is for 2 h at 9V constant voltage. Tank transfer is overnight at 40 V constant voltage.
4. To transfer the gel for blotting, lay a dry piece of Whatman 3MM Chromatography paper over the wet gel. Carefully peel the 3MM paper and gel off the glass plate and immerse, gel side up, in transfer buffer until 3MM paper is thoroughly wet. Remove bubbles by rolling a pipette across the surface of the gel.
5. To confirm detection of p350ATM, a cell line carrying a truncating mutation in ATM should be used as a negative control. Cell lines from A-T patients which show no detectable band at 350 kDa, can be obtained from the National Institute of General Medical Sciences Human Genetic Mutant Cell Repository at the Coriell Institute for Medical Research, 401 Haddon Avenue, Camden, NJ 08103.
6. For immunoprecipitations, prepare nuclear lysates as described. Immunoprecipitate p350ATM using 2 µg of Anti-ATM (Ab-3) Rabbit pAb and Protein A-Agarose (Cat. No. IP06). Detection can be after metabolic labeling with 35S methionine followed by autoradiography, or alternatively, immunoprecipitated proteins can be displayed on 5% SDS/PAGE, transferred to nitrocellulose and then blotted as above using Anti-ATM (Ab-3).
Friedberg, E.C., et al. 1995. Amer. Soc. of Microbiolgy (meeting report), Wash. D.C.
Meyn, S.M. 1995. Cancer Res.55, 5991.
Paules, R.S., et al. 1995. Cancer Res.55, 1763.
Savitsky, K., et al. 1995. Science268, 1749.
Savitsky, K., et al. 1995. Hum. Molec. Genet.4, 2025.
Zakian, V., 1995. Cell82, 685.
Beamish, H., et al. 1993. Rad. Res.138, 130.
Kastan, M.B., et al. 1992. Cell71, 587.
Painter, R.B. and Young, B.R. 1980. Proc. Natl. Acad. Sci. USA77, 7315.
Meyn, S.M. 1995. Cancer Res.55, 5991.
Paules, R.S., et al. 1995. Cancer Res.55, 1763.
Savitsky, K., et al. 1995. Science268, 1749.
Savitsky, K., et al. 1995. Hum. Molec. Genet.4, 2025.
Zakian, V., 1995. Cell82, 685.
Beamish, H., et al. 1993. Rad. Res.138, 130.
Kastan, M.B., et al. 1992. Cell71, 587.
Painter, R.B. and Young, B.R. 1980. Proc. Natl. Acad. Sci. USA77, 7315.
Legal Information
CALBIOCHEM is a registered trademark of Merck KGaA, Darmstadt, Germany
Not finding the right product?
Try our Product Selector Tool.
Storage Class Code
10 - Combustible liquids
WGK
nwg
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
S Faderl et al.
Leukemia, 16(6), 1045-1052 (2002-06-01)
It has been suggested that the expansion of the leukemic cells in chronic lymphocytic leukemia (CLL) is due to dysregulation of pathways of programmed cell death (apoptosis) rather than cell proliferation, although differences may exist in early vs late and
Fengxia Du et al.
Biochemical and biophysical research communications, 452(4), 1034-1039 (2014-09-23)
The ATM protein kinase, is a serine/threonine protein kinase that is recruited and activated by DNA double-strand breaks, mediates responses to ionizing radiation in mammalian cells. Here we show that ATM is held inactive in unirradiated cells as a dimer
Xiaofeng Jiang et al.
The Journal of biological chemistry, 281(23), 15741-15746 (2006-04-11)
Members of the phosphatidylinositol 3-kinase-related kinase (PIKK) family, including the ATM, DNA-PKcs, Atr, and Trrap proteins, function in signal transduction pathways that activate the DNA damage response. PIKK proteins contain a conserved C-terminal FAT/kinase domain/FATC domain structure. The FATC domain
Michael G Kemp et al.
The Journal of biological chemistry, 286(22), 19237-19246 (2011-04-14)
A variety of environmental, carcinogenic, and chemotherapeutic agents form bulky lesions on DNA that activate DNA damage checkpoint signaling pathways in human cells. To identify the mechanisms by which bulky DNA adducts induce damage signaling, we developed an in vitro
Claus Storgaard Sørensen et al.
Cancer cell, 3(3), 247-258 (2003-04-05)
Chk1 kinase coordinates cell cycle progression and preserves genome integrity. Here, we show that chemical or genetic ablation of human Chk1 triggered supraphysiological accumulation of the S phase-promoting Cdc25A phosphatase, prevented ionizing radiation (IR)-induced degradation of Cdc25A, and caused radioresistant
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service