Raw materials precisely tailored to your needs.
Industry leading Pharma/Biopharma raw
materials and expertise tailored to meet your
exact needs.
SAFC® Raw Material Solutions for Pharmaceutical & Biopharmaceutical Manufacturing
The SAFC® portfolio offers Pharma & Biopharma raw materials and expertise tailored to your needs. Our responsive and expert team can support you at every step and stage: cell line and clone selection to final formulation, preclinical to full-scale commercialization.
Bringing pharma and biopharma products to market requires a trusted expert who can support you with exactly the products, services and expertise you need.
Learn more about our products: Bioprocessing & Formulation Raw Materials, Bioprocessing Cell Culture, Formulation Solutions, API & Folate Portfolio.
Consistent, high-quality media, supplements, and reagents for classical and novel therapies that enhance cell growth and productivity and are backed by best-in-class service and regulatory support.
High-quality raw materials, comprehensive documentation, and expert support for your qualification, risk assessment, and process optimization efforts.
Explore our broad range of high quality GMP products for the development and manufacturing of solid, semi-solid and liquid dosage forms for small and large molecule therapeutics.
Comprehensive regulatory support for high-quality active pharmaceutical ingredients (APIs), backed by nearly 200 years of experience. We can support you with new process evaluation, product registration and filing, process development and validation, reference standard synthesis, and regulatory filing.
BioProcessing Cell Culture
CHOZN® CHO Expression System
A CHO platform for fast, easy, metabolic-free selection and scale-up of clones for manufacturing.
The CHOZN® platform includes CHO cell lines, paired media & feeds, and optimized protocols that result in predictable, stable high titers. This platform is fully supported with quality documentation, regulatory-friendly ZFN gene editing technology, and expert consultation services.

CHO Cell Culture Media & Feeds
Chemically defined, ready-to-use CHO media designed for commercial fed-batch and perfusion processes – enabling high titers, robust scalability, superior protein quality, and accelerated time to market.
Rely on Cellvento® and EX-CELL® Advanced media products for:
- Consistent high cell growth and productivity
- Increased productivity with the right feed combination
- Proven success in batch, fed-batch and perfusion modes
- Comprehensive documentation and support

ImMEDIAte ADVANTAGE® Lab Services
We support biopharmaceutical manufacturing and process development with expedited timing of small volume custom cell culture media recipes.
- We formulate cell culture media to your specification
- Our fast-track services accelerate media development and optimization timelines
- Our compounding methods and qualified raw materials ensure consistency and scalability of your formulation to full-scale GMP manufacturing

Compacted Cell Culture Media
Designed to meet your need for ease-of-use, convenience and operator safety.
Our compacted raw materials and cell culture media, including Cellvento® 4CHO and Cellvento® 4Feed, are dust-free, ready-to-use materials with increased flowability for better handling, safety and processing. For cell culture media the advantage of compaction is in the significant reduction of dissolution time.

EZ BioPAC® Dry Powder Bags
& Right Sized Weighing
Simplifies bulk powder transfers and additions, for all scales of operation, making operation faster, cleaner and safer.
- Enables a single worker to easily dispense specific quantities of media and buffer powders
- Minimizes product contamination risk and safeguards employees
- Increases process security and efficiency

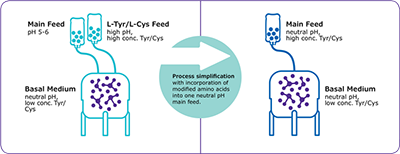
Modified Amino Acids
(phospho-tyrosine, sulfo-cystine)
Phospho-L-Tyrosine disodium salt and S-Sulfocysteine sodium salt can be used together, as a single addition, enabling highly concentrated, pH neutral feeds.
- Simplifies fed-batch processes and reduces pH spikes
- Enables the intensification of fed-batch cell culture processes
- Allows for room-temperature-stable formulations
SAFC® Cell Culture Solutions
Upstream process development and cGMP solutions tailored to your needs and fully backed by regulatory documentation and support.
BioProcessing & Formulation Raw Materials
High Temperature Short Time (HTST) Treated Glucose
Prevent viral contamination of cell culture media feeds with High Temperature Short Time (HTST) Treated Glucose.
- Provides a point-of-origin solution to mitigate the risk of viral contamination
- Enables robust clearance of viruses
- Ready-to-use, fast, sterile filtered, scalable
Benzonase® Endonuclease
Ensures efficient and compliant DNA and RNA removal in biopharmaceutical production across a wide range of operating conditions and applications, including:
- Purifying viral vectors with Benzonase® endonuclease Safety Plus Emprove® Expert
- Purifying viral vaccines, proteins and other biologics with Benzonase® endonuclease Emprove® bio
- Reducing biofouling and viscosity caused by nucleic acids
Benzonase® enzymatically degrades all forms of DNA and RNA without impacting proteolytic activity and includes our Emprove® documentation to support your regulatory filing— ensuring compliance, consistent quality and higher yields.

Modified Amino Acids
(phospho-tyrosine, sulfo-cystine)
Phospho-L-Tyrosine disodium salt and S-Sulfocysteine sodium salt can be used together, as a single addition, enabling highly concentrated, pH neutral feeds.
- Simplifies fed-batch processes and reduces pH spikes
- Enables the intensification of fed-batch cell culture processes
- Allows for room-temperature-stable formulations

SAFC® Critical Process Chemicals
USP & DSP process chemicals in a range of quality grades, formats and packaging options, supported by our technical and regulatory experts and full documentation.
API & Folate Portfolio
High Potency & Complex APIs
Full portfolio of standard and custom raw material solutions for pharma and biologics; spanning the entire drug development process - from chemical and biological API synthesis to final formulation

Metafolin®/Arcofolin™ L-Methylfolate
Metafolin® and Arcofolin™ L-Methylfolate are the superior forms of folate supplementation as they are directly bioavailable, offering an excellent stability, high solubility and superior bioavailability compared to folic acid.
- Arcofolin™ provides a higher level of active folate, high purity and improved solubility in water.
- Developed by experts with 60 years of experience in the field of reduced folates and manufactured in a state-of-the-art facility with an outstanding cGMP track record.

Formulation Solutions
Parteck® MXP Functional Excipient
Polyvinyl alcohol-based excipient for hot melt extrusion (HME) to solve formulation challenges with low solubility APIs.
- Fully compliant with US, EU and JPE pharmacopeias.
- Enhances API solubility
- Enables stable drug loads of 30% or more for a broad range of APIs
- Features a high thermostability (above 200 °C), thus broadening the applicable API range

Granulated products for liquids Glycine Emprove® Expert
GMP-grade excipient with increased flowability for better handling, safety and processing — designed to meet your need for ease-of-use, convenience and operator safety
- Reduces caking behavior
- Provides a ready-to-use, dust-free material
- Fully compliant with Ph. Eur., BP, JPE and USP
- Supported with Emprove® documentation and EXCiPACT™ certificate
Meglumine Excipient
Meglumine improves API solubility, bioavailability and stability in solid and liquid formulations.
Our Emprove® API and Emprove® Essential grades meet the highest quality standards and the needs of drug manufacturers, regulatory authorities, and patient.
SynBiosys® Polymer Platform for sustained release of biologics
Reduce patient drug-dosing frequency with a new polymeric drug delivery technology that enables sustained release formulations for virtually any class of biological API.
- Reduces dosing frequency and maintains drugs at therapeutic levels longer, to improve patient compliance
- Suitable for large biological active pharmaceutical ingredients
- Scalable and easily adjustable to specific requirements
- Clinically proven safe

Emprove® Program and Dossiers: Full supply transparency
The SAFC® portfolio of more than 400 raw and starting materials provides comprehensive documentation which addresses current regulatory requirements and anticipates future ones.
Emprove® dossiers simplify and speed your processes by:
- Accelerating approval preparation
- Increasing supply transparency and facilitating qualification processes
- Supporting risk assessment, management and mitigation plans

mPredict™: Co-Crystal Prediction Service – Enhancing the Solubility of APIs with AI
AI-Powered Solutions for Faster Drug Formulation
- Predicts formulate-ability early in drug development
- Lowers experimental and API requirements
- Leverages AI to help you formulate new APIs more efficiently

SAFC® Formulation Excipients
An extensive portfolio of excipients and drug delivery solutions to help accelerate your journey to market - supported by comprehensive regulatory dossiers.
Value Added Services

Emprove® Program the Smart Way to Master Compliance and Control
Complementing our product portfolio, the Emprove® Program provides convenient access to reliable and detailed technical, regulatory and supply information in Emprove® Dossiers to support your risk assessment continuum and streamline the regulatory approval process.
Critical Raw Materials
Our customized portfolio of high-quality critical raw materials supplies the documentation, and expertise you need to meet stringent regulatory requirements and mitigate risk in all aspects of drug development and manufacturing.
Custom Media Development Services
Our Media Development Services will identify a custom media solution that meets your objectives for cell growth, titer, protein quality profile, as well as media, feed, and perfusion requirements.
Security and Sustainability

Sustainability
We are committed to reducing environmental impact and advancing sustainable scientific practices through the development of greener products, energy-efficient processes, recycling, sustainable packaging and waste reduction strategies.
Security of Supply
We know you need a supplier focused on supply robustness and resiliency, that can meet your changing needs and help you prepare for changing regulations and shift to more sustainable business operations. Our multi-faceted and global approach includes:
- Business Continuity Planning and Disaster Recovery Planning
- Supplier Risk Management
- Supply Chain Mapping
WE BRING TOGETHER 6 OF THE WORLD’S LEADING LIFE SCIENCE BRANDS TO HELP YOU SOLVE THE GREATEST CHALLENGES IN LIFE SCIENCE.
Lab & Production Materials
Expert Pharma/ BioPharma Products & CTDMO Services
Analytical Products
Lab Water Solutions
Contract Testing Services
To continue reading please sign in or create an account.
Don't Have An Account?