S9882
Sulfachloropyridazine
동의어(들):
4-Amino-N-(6-chloro-3-pyridazinyl)benzenesulfonamide, N1-(6-Chloro-3-pyridazinyl)sulfanilamide
About This Item
추천 제품
양식
powder
색상
off-white to light yellow
solubility
0.5 M NaOH: soluble 50 mg/mL
항생제 활성 스펙트럼
Gram-negative bacteria
Gram-positive bacteria
mycoplasma
동작 모드
DNA synthesis | interferes
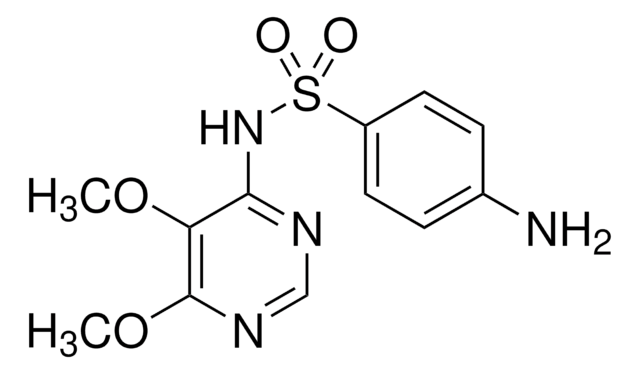
SMILES string
Nc1ccc(cc1)S(=O)(=O)Nc2ccc(Cl)nn2
InChI
1S/C10H9ClN4O2S/c11-9-5-6-10(14-13-9)15-18(16,17)8-3-1-7(12)2-4-8/h1-6H,12H2,(H,14,15)
InChI key
XOXHILFPRYWFOD-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
애플리케이션
생화학적/생리학적 작용
기타 정보
이미 열람한 고객
활성 필터
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.