모든 사진(1)
About This Item
실험식(Hill 표기법):
C217H341N71O74S9
CAS Number:
Molecular Weight:
5417.05
MDL number:
UNSPSC 코드:
12352200
가격 및 재고 정보를 현재 이용할 수 없음
추천 제품
생물학적 소스
Echis carinatus
분석
≥90% (HPLC)
양식
powder
기술
flow cytometry: suitable
inhibition assay: suitable
저장 온도
−20°C
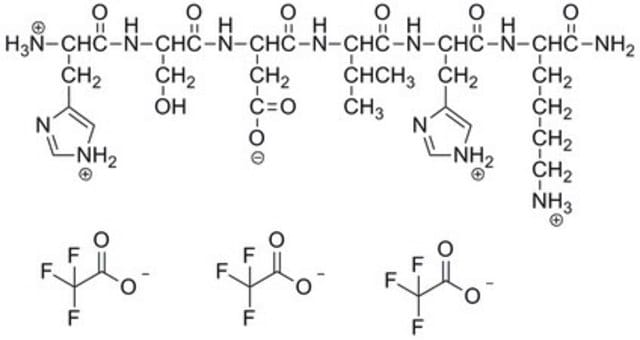
Amino Acid Sequence
Glu-Cys-Glu-Ser-Gly-Pro-Cys-Cys-Arg-Asn-Cys-Lys-Phe-Leu-Lys-Glu-Gly-Thr-Ile-Cys-Lys-Arg-Ala-Arg-Gly-Asp-Asp-Met-Asp-Asp-Tyr-Cys-Asn-Gly-Lys-Thr-Cys-Asp-Cys-Pro-Arg-Asn-Pro-His-Lys-Gly-Pro-Ala-Thr
일반 설명
Echistatin is a single chain 49 amino acid residue protein, which prevents the aggregation of platelets. It has an isoelectric point (pI) of 8.3 and a molecular weight of 5400. This peptide is present in the venom of Echis carinatus, which is a saw-scaled viper. It contains the arginine-glycine-aspartic (RGD) acid sequence, which is present in proteins that bind to glycoprotein IIb/IIIa complex. It shares the proline-arginine-asparagine-proline sequence with the Aα chain of human fibrinogen.[1] This protein is a member of disintegrin family, which prevents cell adhesion.[2] With regards to molecular weight, echistatins are the smallest member of disintegrin family, and contains four isoforms called, α1, α2, β and γ.[3]
애플리케이션
Echistatin has been used:
- as an inhibitor of integrin function to study the role of microfibril-associated glycoprotein-1 (Magp1) in the morphogenesis of vascular structures[4]
- for the preparation of microbubbles targeted to αvβ3 integrins in tumor angiogenesis imaging[5]
- as a conjugate to αvβ3 in flow cytometric binding studies[6]
생화학적/생리학적 작용
A member of the disintegrins family, a novel family of integrin β1 and β3 inhibitor proteins and the most potent known inhibitors of integrin function.
Disintegrins represent a novel family of integrin β1 and β3 inhibitor proteins isolated from viper venoms. They are low molecular-weight, cysteine-rich peptides containing the Arg-Gly-Asp (RGD) sequence. They are the most potent known inhibitors of integrin function. Disintegrins interfere with cell adhesion to the extracellular matrix, including adhesion of melanoma cells and fibroblasts to fibronectin, and are potent inhibitors of platelet aggregation.
Echistatin is a disintegrin, which prevents the aggregation of platelets. They interact with and prevent the binding of fibrinogen to their receptors on the membrane of platelets.[3] It also inhibits platelet aggregation mediated by epinephrine, thrombin, collagen, or platelet-activating factor.[1] Studies in isolated osteoclasts show that this peptide inhibits bone resorption by osteoclasts, most probably by damaging adhesion structures.[7]
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
C C Kumar et al.
The Journal of pharmacology and experimental therapeutics, 283(2), 843-853 (1997-11-14)
Echistatin is a 49-amino-acid peptide belonging to the family of disintegrins that are derived from snake venoms and are potent inhibitors of platelet aggregation and cell adhesion. Integrin alphavbeta3 receptor plays a critical role in several physiological processes such as
Dilantha B Ellegala et al.
Circulation, 108(3), 336-341 (2003-07-02)
Angiogenesis is a critical determinant of tumor growth and metastasis. We hypothesized that contrast-enhanced ultrasound (CEU) with microbubbles targeted to alpha(v)-integrins expressed on the neovascular endothelium could be used to image angiogenesis. Malignant gliomas were produced in 14 athymic rats
L C Chuang et al.
Biochemical and biophysical research communications, 220(2), 246-254 (1996-03-18)
An echistatin analogue, designated as des(46-49)-[Ala8,37]-echistatin gamma, was synthesized chemically by solid-phase peptide synthesis. The analogue was made by replacing Cys8 and Cys37 residues with two alanines and the deletion of C-terminal peptide 46-49 of echistatin gamma, resulting in an
M Sato et al.
The Journal of cell biology, 111(4), 1713-1723 (1990-10-01)
The venom protein, s-echistatin, originally derived from the saw-scaled viper Echis carinatus, was found to be a potent inhibitor of bone resorption by isolated osteoclasts. This Arg24-Gly25-Asp26-(RGD)-containing protein inhibited the excavation of bone slices by rat osteoclasts (IC50 = 0.1
M Gawaz et al.
Circulation, 96(6), 1809-1818 (1997-10-10)
Platelet interaction with endothelium plays an important role in the pathophysiology of coronary microcirculation. We assessed the role of the vitronectin receptor (integrin alpha(v)beta3) in platelet/endothelium adhesion. We investigated the effect on platelet/endothelium adhesion of plasma obtained from patients with
활성 필터
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.