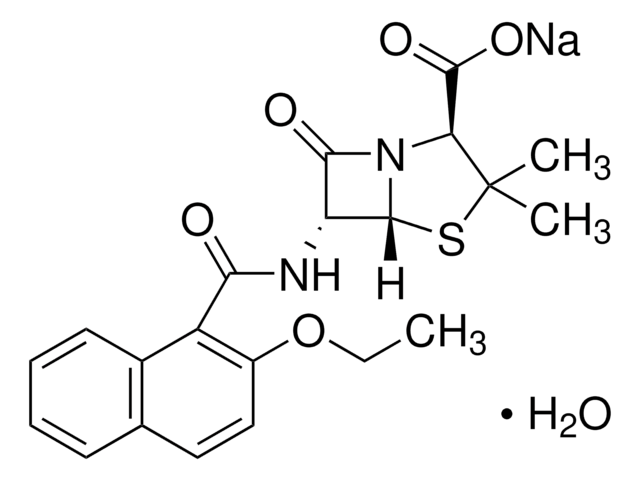
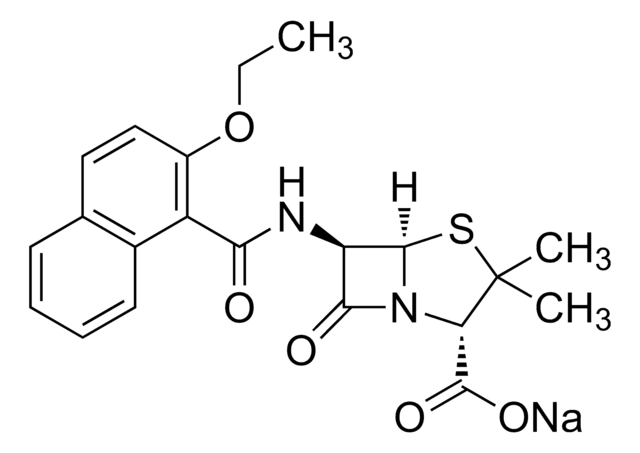
D9016
Dicloxacillin sodium salt monohydrate
동의어(들):
3-(2,6-Dichlorophenyl)-5-methyl-4-isoxazolyl penicillin sodium salt monohydrate
About This Item
추천 제품
형태
powder or crystals
Quality Level
색상
white to off-white
solubility
H2O: 100 mg/mL
항생제 활성 스펙트럼
Gram-negative bacteria
Gram-positive bacteria
동작 모드
cell wall synthesis | interferes
저장 온도
2-8°C
SMILES string
O.[Na+].Cc1onc(c1C(=O)N[C@H]2[C@H]3SC(C)(C)[C@@H](N3C2=O)C([O-])=O)-c4c(Cl)cccc4Cl
InChI
1S/C19H17Cl2N3O5S.Na.H2O/c1-7-10(12(23-29-7)11-8(20)5-4-6-9(11)21)15(25)22-13-16(26)24-14(18(27)28)19(2,3)30-17(13)24;;/h4-6,13-14,17H,1-3H3,(H,22,25)(H,27,28);;1H2/q;+1;/p-1/t13-,14+,17-;;/m1../s1
InChI key
SIGZQNJITOWQEF-VICXVTCVSA-M
유사한 제품을 찾으십니까? 방문 제품 비교 안내
관련 카테고리
일반 설명
애플리케이션
생화학적/생리학적 작용
신호어
Danger
유해 및 위험 성명서
예방조치 성명서
Hazard Classifications
Eye Irrit. 2 - Resp. Sens. 1 - Skin Irrit. 2 - STOT SE 3
표적 기관
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
dust mask type N95 (US), Eyeshields, Faceshields, Gloves
시험 성적서(COA)
제품의 로트/배치 번호를 입력하여 시험 성적서(COA)을 검색하십시오. 로트 및 배치 번호는 제품 라벨에 있는 ‘로트’ 또는 ‘배치’라는 용어 뒤에서 찾을 수 있습니다.
이미 열람한 고객
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.