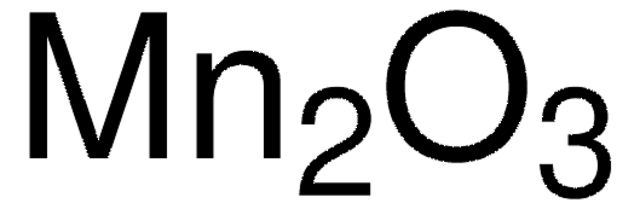추천 제품
일반 설명
Manganese(IV) oxide is an oxidizing reagent that can be used for the oxidation of propargylic alcohols, benzylic or heterocyclic alcohols, saturated alcohols, 1,2-diols, allylic alcohols to α, β-ethylenic aldehydes or ketones, and amines to aldehydes, imines, amides, and diazo compounds. It can also be used for the conversion of allylic alcohols to α, β-ethylenic esters or amides, hydration of nitriles to amides, dehydrogenation and aromatization reactions.
애플리케이션
- High-oxidation-state 3d metal complexes: Explores the catalytic properties of manganese(IV) oxide within high-oxidation-state complexes for advanced organic synthesis, demonstrating its critical role in accelerating chemical reactions and enhancing yield efficiencies, beneficial for pharmaceutical and chemical industries (Cheng J et al., 2018).
- Synthesis and properties of manganese complexes: Details the synthesis of new manganese complexes that demonstrate unique redox properties, useful for understanding electron transfer processes in various chemical and environmental contexts (Baffert C et al., 2002).
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - STOT RE 2 Inhalation
표적 기관
Brain
Storage Class Code
13 - Non Combustible Solids
WGK
WGK 2
Flash Point (°F)
does not flash
Flash Point (°C)
does not flash
시험 성적서(COA)
제품의 로트/배치 번호를 입력하여 시험 성적서(COA)을 검색하십시오. 로트 및 배치 번호는 제품 라벨에 있는 ‘로트’ 또는 ‘배치’라는 용어 뒤에서 찾을 수 있습니다.
이미 열람한 고객
Manganese dioxide
Cahiez G, et al.
e-EROS Encyclopedia of Reagents for Organic Synthesis (2001)
Y Wang et al.
Journal of colloid and interface science, 380(1), 8-15 (2012-06-02)
Bio-inspired chemical approach has been developed for the surface modification and electrophoretic deposition of manganese dioxide and zirconia nanoparticles, prepared by chemical precipitation methods. Caffeic acid, trans-cinnamic acid, p-coumaric acid, and 2,4-dihydroxycinnamic acid were investigated for the surface modification of
Wen-Hui Kuan et al.
Journal of hazardous materials, 239-240, 152-159 (2012-09-25)
This study examined the reaction of methylene blue (MB) with tunneled manganese oxide pyrolusite regarding pH and reaction time. MB was cleaved through N-demethylation, in which reaction azure B (AB), azure A (AA), azure C (AC), and thionin (TH) were
Michael K L Chu et al.
Lab on a chip, 12(14), 2533-2539 (2012-05-09)
We have developed glucose-responsive implantable microdevices for closed-loop delivery of insulin and conducted in vivo testing of these devices in diabetic rats. The microdevices consist of an albumin-based bioinorganic membrane that utilizes glucose oxidase (GOx), catalase (CAT) and manganese dioxide
Xin Zhao et al.
ACS nano, 6(6), 5404-5412 (2012-05-05)
Manganese dioxide (MnO(2)) particles 2-3 nm in size were deposited onto a porous "activated microwave expanded graphite oxide" (aMEGO) carbon scaffold via a self-controlled redox process. Symmetric electrochemical capacitors were fabricated that yielded a specific capacitance of 256 F/g (volumetric:
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.