추천 제품
Grade
pharmaceutical primary standard
API family
gliclazide
형태
crystalline powder
유통기한
limited shelf life, expiry date on the label
제조업체/상표
BP
mp
163-169 °C (lit.)
응용 분야
pharmaceutical
pharmaceutical small molecule
형식
neat
저장 온도
2-8°C
SMILES string
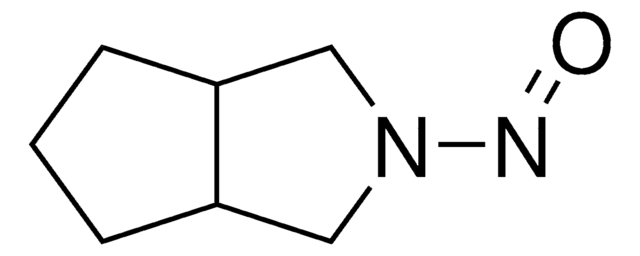
Cc1ccc(cc1)S(=O)(=O)NC(=O)NN2CC3CCCC3C2
InChI
1S/C15H21N3O3S/c1-11-5-7-14(8-6-11)22(20,21)17-15(19)16-18-9-12-3-2-4-13(12)10-18/h5-8,12-13H,2-4,9-10H2,1H3,(H2,16,17,19)
InChI key
BOVGTQGAOIONJV-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the issuing Pharmacopoeia.For further information and support please go to the website of the issuing Pharmacopoeia.
애플리케이션
Gliclazide BP Reference standard, intended for use in laboratory tests only as specifically prescribed in the British Pharmacopoeia.
Also used in monographs such as:Gliclazide Tablets
Also used in monographs such as:
Used in the treatment of non-insulin dependent diabetes mellitus (NIDDM).
생화학적/생리학적 작용
Oxidative modification of low-density lipoprotein (LDL) plays an important role in vascular dysfunction associated with diabetes mellitus. Gliclazide is a second-generation sulfonylurea with free-radical-scavenging activity. Incubation of human aortic smooth muscle cell (HASMC) with native human LDL (100 μg/mL) in the presence of increasing concentrations of gliclazide (1 to 10 μg/mL) resulted in a dose-dependent decrease in HASMC-mediated LDL oxidation. Exposure of HASMCs to gliclazide (1 to 10 μg/mL) and native LDL (100 μg/mL) also led to a dose-dependent decrease in oxidized LDL-induced human monocyte adhesion to HASMCs. In addition, incubation of HASMCs with gliclazide dramatically reduced the ability of oxidized LDL to stimulate the proliferation of these cells. Finally, treatment of HASMCs with gliclazide resulted in a marked decrease in oxidatively modified LDL-induced monocyte chemoattractant protein (MCP)-1 and human heat shock protein 70 (HSP 70) expression, both at the gene and protein levels. These results show that gliclazide, at concentrations in the therapeutic range (5 to 10 μg/mL), is effective in vitro in reducing vascular smooth muscle cell (VSMC) dysfunction induced by oxidatively modified LDL. Administration of gliclazide to type 2 diabetic patients could form part of the strategy for the prevention and management of diabetic cardiovascular diseases
포장
Unit quantity: 200 mg. Subject to change. The product is delivered as supplied by the issuing Pharmacopoeia. For the current unit quantity please visit British Pharmacopoeia
기타 정보
Sales restrictions may apply.
관련 제품
제품 번호
설명
가격
신호어
Warning
유해 및 위험 성명서
예방조치 성명서
Hazard Classifications
Acute Tox. 4 Oral
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
Choose from one of the most recent versions:
시험 성적서(COA)
Lot/Batch Number
Sorry, we don't have COAs for this product available online at this time.
If you need assistance, please contact 고객 지원 부서
이미 열람한 고객
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.