추천 제품
vapor pressure
1 mmHg ( 104 °C)
Quality Level
분석
98%
형태
powder or crystals
autoignition temp.
890 °F
bp
293 °C (lit.)
mp
65-68 °C (lit.)
density
1.09 g/mL at 25 °C (lit.)
응용 분야
diagnostic assay manufacturing
hematology
histology
저장 온도
room temp
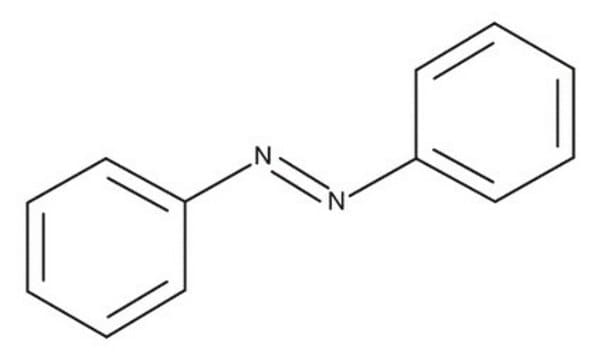
SMILES string
c1ccc(cc1)\N=N\c2ccccc2
InChI
1S/C12H10N2/c1-3-7-11(8-4-1)13-14-12-9-5-2-6-10-12/h1-10H/b14-13+
InChI key
DMLAVOWQYNRWNQ-BUHFOSPRSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
Azobenzene is a chromogen and an azo compound that exhibits trans/cis isomerism yielding isomers that differ in color. It is a simple azoarene compound that shows cis-trans isomerization around the azo bond in response to light.
애플리케이션
Azobenzene and its derivatives have multiple applications in the field of mechanical and optical materials, in photopharmacology, and molecular photo-switches including in biological probes.
As human tissue is translucent to red and near-infrared light but opaque to blue and UV light, Azobenzene is important in medicine and photopharmacology for applications that involve shifting the absorptions of both trans and cis isomers of azobenzene to longer wavelengths.
As human tissue is translucent to red and near-infrared light but opaque to blue and UV light, Azobenzene is important in medicine and photopharmacology for applications that involve shifting the absorptions of both trans and cis isomers of azobenzene to longer wavelengths.
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Aquatic Acute 1 - Aquatic Chronic 1 - Carc. 1B - Muta. 2 - STOT RE 2
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Flash Point (°F)
212.0 °F - closed cup
Flash Point (°C)
100.0 °C - closed cup
개인 보호 장비
Eyeshields, Gloves, type P3 (EN 143) respirator cartridges
이미 열람한 고객
Michael S Scholz et al.
The journal of physical chemistry. A, 121(34), 6413-6419 (2017-08-05)
Because of their high photoisomerization efficiencies, azobenzenes and their functionalized derivatives are used in a broad range of molecular photoswitches. Here, the photochemical properties of the trans isomers of protonated azobenzene (ABH
P S Ramanujam et al.
Optics express, 21(2), 1812-1819 (2013-02-08)
We demonstrate a new type of anisotropy in thin films of amorphous azobenzene polymers induced between 570 and 633 nm, where the absorbance in the film is on the order of 0.05. The anisotropy has a pronounced radial contribution. This
Fastest thermal isomerization of an azobenzene for nanosecond photoswitching applications under physiological conditions.
Jaume Garcia-Amorós et al.
Angewandte Chemie (International ed. in English), 51(51), 12820-12823 (2012-11-13)
Daisuke Ishikawa et al.
Langmuir : the ACS journal of surfaces and colloids, 29(14), 4622-4631 (2012-12-20)
The growth processes of self-assembled monolayers (SAMs) of two azobenzene disulfides formed on flat gold surfaces were studied to confirm the effect of the intermolecular interactions between azobenzene molecules on the light-triggered surface morphologies of the SAMs. Scanning tunneling microscopy
C Renner et al.
The journal of peptide research : official journal of the American Peptide Society, 65(1), 4-14 (2005-02-03)
Over the last decades azobenzene has been the most widely used optical trigger for the synthesis of photoresponsive systems ranging from poly-alpha-amino acids to innovative materials with light-controlled mechanical and optical properties. More recently, its use in form of appropriate
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.