추천 제품
Grade
analytical standard
유통기한
limited shelf life, expiry date on the label
기술
HPLC: suitable
gas chromatography (GC): suitable
bp
242 °C (lit.)
mp
98-100 °C (lit.)
응용 분야
environmental
형식
neat
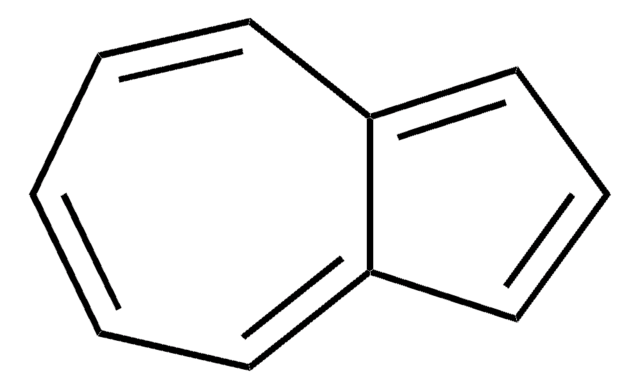
SMILES string
c1ccc2cccc2cc1
InChI
1S/C10H8/c1-2-5-9-7-4-8-10(9)6-3-1/h1-8H
InChI key
CUFNKYGDVFVPHO-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
애플리케이션
Refer to the product′s Certificate of Analysis for more information on a suitable instrument technique. Contact Technical Service for further support.
추천 제품
Find a digital Reference Material for this product available on our online platform ChemisTwin® for NMR. You can use this digital equivalent on ChemisTwin® for your sample identity confirmation and compound quantification (with digital external standard). An NMR spectrum of this substance can be viewed and an online comparison against your sample can be performed with a few mouseclicks. Learn more here and start your free trial.
유해 및 위험 성명서
예방조치 성명서
Hazard Classifications
Aquatic Chronic 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Mine Ince et al.
Chemical communications (Cambridge, England), 48(34), 4058-4060 (2012-03-22)
A novel supramolecular electron donor-acceptor hybrid (2·1) and an electron donor-acceptor conjugate (3), both exhibiting a remarkably shifted Q band in the NIR region of the solar spectrum, were prepared. Irradiation of the supramolecular ensemble 2·1 within the visible range
Dawei Zhao et al.
The Journal of chemical physics, 131(18), 184307-184307 (2009-11-18)
The infrared (IR) spectrum of protonated azulene (AzuH(+), C(10)H(9)(+)) has been measured in the fingerprint range (600-1800 cm(-1)) by means of IR multiple photon dissociation (IRMPD) spectroscopy in a Fourier transform ion cyclotron resonance mass spectrometer equipped with an electrospray
Stefan Löber et al.
ChemMedChem, 4(3), 325-328 (2009-02-03)
Blue makes it happen: The non-uniform charge distribution of the blue colored azulene framework is highly suitable for the bioisosteric replacement of bicyclic heteroarene moieties. Showing an analogous binding mode as heterocyclic dopamine D4 receptor-selective lead compounds, the induction of
Atsuya Muranaka et al.
Journal of the American Chemical Society, 132(23), 7844-7845 (2010-05-27)
Azulenocyanine, having four azulene units fused to a tetraazaporphyrin skeleton, is a structural isomer of naphthalocyanine. We synthesized the first example of an azulenocyanine from 1,3-di-tert-butyl-5,6-dicyanoazulene. The macrocycle exhibits broad absorption over the visible and near-IR regions far beyond 1000
Timothy D Lash et al.
The Journal of organic chemistry, 77(5), 2368-2381 (2012-02-01)
A "2 + 2" strategy for synthesizing adj-dicarbaporphyrinoid systems has been developed. In a model study, an azulenylmethylpyrrole dialdehyde was condensed with a dipyrrylmethane in the presence of HCl, followed by oxidation with ferric chloride, to give a modest yield
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.