추천 제품
생물학적 소스
mouse
Quality Level
항체 형태
purified immunoglobulin
항체 생산 유형
primary antibodies
클론
3F4, monoclonal
종 반응성
hamster, human
제조업체/상표
Chemicon®
기술
ELISA: suitable
immunohistochemistry (formalin-fixed, paraffin-embedded sections): suitable
immunoprecipitation (IP): suitable
western blot: suitable
동형
IgG2a
NCBI 수납 번호
UniProt 수납 번호
배송 상태
dry ice
타겟 번역 후 변형
unmodified
유전자 정보
human ... PRNP(5621)
일반 설명
Prions are thought to cause a number of diseases in a variety of mammals, including bovine spongiform encephalopathy (BSE, also known as "mad cow disease") in cattle and the Creutzfeldt-Jakob disease (CJD) in humans. All thus-far hypothesized prion diseases affect the structure of the brain or other neural tissue, and all are currently untreatable and thought to be fatal. Prions are hypothesized to infect and propagate by refolding abnormally into a structure which is able to convert normal molecules of the protein into the abnormally structured form. All known prions induce the formation of an amyloid fold, in which the protein polymerises into an aggregate consisting of tightly packed beta sheets. This altered structure is extremely stable and accumulates in infected tissue, causing cell death and tissue damage. This stability means that prions are resistant to denaturation by chemical and physical agents, making disposal and containment of these particles difficult.
특이성
Prion protein, amino acid residues 109-112 of human, hamster and feline. Does not react with PrP from any other mammalian species. MAB1562 is reactive to native and denatured forms of PrP. Tissue or cells which have been fixed requires that the epitope be re-exposed (see below). Recognizes both protease sensitive and protease resistant forms of PrP.
면역원
Epitope: a.a. 109-112
애플리케이션
Immunohistochemistry(paraffin):
Representative images from a previous lot. Optimal Staining With Citrate Buffer, pH 6.0, Epitope Retrieval: Human Brain
Immunohistochemistry (Kitamoto et al., 1987):
1:100-1:1,000 *See protocol below.
Epitope must be re-exposed in fixed tissue by pretreatment of tissue using one of the following procedures:
a. formic acid for 10 minutes at room temperature (Kitamoto et al., 1987)
b. hydrolytic autoclaving (Kitamoto et al., 1991)
c. microwaving (BioGenex, San Ramon, CA)
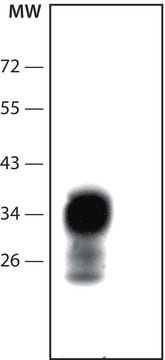
Western Blot: (Kascsak, R.J., 1991; Kascsak, R.J., 1987):
1:10,000-1:100,000 dilution of a previous lot was used.
Immunoprecipitation: (Kascsak, R.J., 1991; Kascsak, R.J., 1987):
1:10-1:100 dilution of a previous lot was used.
ELISA: (Kascsak, R.J., 1991; Kascsak, R.J., 1987):
1:100,000 dilution of a previous lot was used.
Optimal working dilutions must be determined by end user.
Representative images from a previous lot. Optimal Staining With Citrate Buffer, pH 6.0, Epitope Retrieval: Human Brain
Immunohistochemistry (Kitamoto et al., 1987):
1:100-1:1,000 *See protocol below.
Epitope must be re-exposed in fixed tissue by pretreatment of tissue using one of the following procedures:
a. formic acid for 10 minutes at room temperature (Kitamoto et al., 1987)
b. hydrolytic autoclaving (Kitamoto et al., 1991)
c. microwaving (BioGenex, San Ramon, CA)
Western Blot: (Kascsak, R.J., 1991; Kascsak, R.J., 1987):
1:10,000-1:100,000 dilution of a previous lot was used.
Immunoprecipitation: (Kascsak, R.J., 1991; Kascsak, R.J., 1987):
1:10-1:100 dilution of a previous lot was used.
ELISA: (Kascsak, R.J., 1991; Kascsak, R.J., 1987):
1:100,000 dilution of a previous lot was used.
Optimal working dilutions must be determined by end user.
This Anti-Prion Protein Antibody, a.a. 109-112, clone 3F4 is validated for use in ELISA, IH, IH(P), IP, WB for the detection of Prion Protein.
품질
Immunohistochemistry(paraffin):
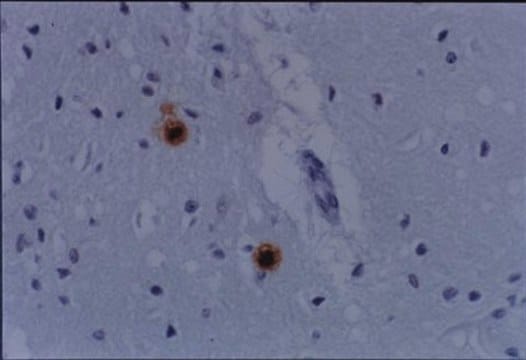
Prion Protein (cat. # MAB1562) staining pattern/morphology in normal brain. Tissue was pretreated with Citrate, pH 6.0. This lot of antibody was diluted to 1:500, using IHC-Select Detection with HRP-DAB. Immunoreactivity is seen predominantly as cell body staining of neurons.
Optimal Staining With Citrate Buffer, pH 6.0, Epitope Retrieval: Human Brain
Prion Protein (cat. # MAB1562) staining pattern/morphology in normal brain. Tissue was pretreated with Citrate, pH 6.0. This lot of antibody was diluted to 1:500, using IHC-Select Detection with HRP-DAB. Immunoreactivity is seen predominantly as cell body staining of neurons.
Optimal Staining With Citrate Buffer, pH 6.0, Epitope Retrieval: Human Brain
표적 설명
12.3 kDa
물리적 형태
Format: Purified
Purified mouse monoclonal IgG2a in buffer containing PBS and no preservative.
법적 정보
CHEMICON is a registered trademark of Merck KGaA, Darmstadt, Germany
Not finding the right product?
Try our 제품 선택기 도구.
Storage Class Code
12 - Non Combustible Liquids
WGK
WGK 2
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
시험 성적서(COA)
제품의 로트/배치 번호를 입력하여 시험 성적서(COA)을 검색하십시오. 로트 및 배치 번호는 제품 라벨에 있는 ‘로트’ 또는 ‘배치’라는 용어 뒤에서 찾을 수 있습니다.
Jacob I Ayers et al.
PLoS pathogens, 7(3), e1001317-e1001317 (2011-03-26)
Prion strains are characterized by differences in the outcome of disease, most notably incubation period and neuropathological features. While it is established that the disease specific isoform of the prion protein, PrP(Sc), is an essential component of the infectious agent
Qi Shi et al.
International journal of molecular medicine, 41(4), 2413-2419 (2018-02-03)
Normal prion protein (PrP) contains two cysteines at amino acids 179 and 214, which may form intra‑ and interpeptide disulfide bonds. To determine the possible effects of this disulfide bridge on the biochemical features of PrP, prokaryotic recombinant human wild‑type PrP
Zuzana Krejciova et al.
The Journal of experimental medicine, 214(12), 3481-3495 (2017-11-17)
Prions are infectious agents that cause neurodegenerative diseases such as Creutzfeldt-Jakob disease (CJD). The absence of a human cell culture model that replicates human prions has hampered prion disease research for decades. In this paper, we show that astrocytes derived
Michele Christine Landemberger et al.
Journal of neurochemistry, 145(5), 409-416 (2018-01-18)
Cellular prion protein (PrPC ) is widely expressed and displays a variety of well-described functions in the central nervous system (CNS). Mutations of the PRNP gene are known to promote genetic human spongiform encephalopathies, but the components of gain- or
Phospholipid composition of membranes directs prions down alternative aggregation pathways.
Robinson, PJ; Pinheiro, TJ
Biophysical Journal null
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.