추천 제품
Quality Level
제품 라인
Novabiochem®
분석
≥95.0% (HPLC)
≥97% (TLC)
형태
powder
반응 적합성
reaction type: Fmoc solid-phase peptide synthesis
제조업체/상표
Novabiochem®
응용 분야
peptide synthesis
작용기
carboxylic acid
저장 온도
15-25°C
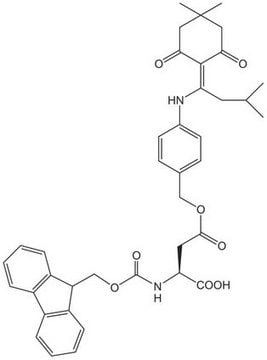
일반 설명
Quasi-orthogonally-protected Asp derivative.The Dmab group can be removed selectively in the presence of tBu-based protecting groups by treatment with 2% hydrazine in DMF [1], making this derivative an extremely useful tool for the preparation of cyclic peptides by Fmoc SPPS or for library synthesis. Occasionally sluggish cleavage of the aminobenzyl moiety is observed [2,3]. In these instances, washing the support with 20% DIPEA in DMF/water (9:1) [2] or HCl in dioxane [4] has been found to be efficacious. For the on-resin synthesis of side-chain to side-chain lactam bridged peptides, the combination of Lys(ivDde) and Asp(ODmab) is particularly advantageous since both side-chains can be simultaneously unmasked in a single step.To avoid aspartimide formation, it is advisable to employ an Hmb- or Dmb-protected derivative for introduction of the preceding residue.For applications of this derivative in the synthesis of cyclic peptides, see references [5 - 7].
Associated Protocols and Technical Articles
Cleavage and Deprotection Protocols for Fmoc SPPS
Literature references
[1] W. C. Chan, et al. (1995) J. Chem. Soc., Chem. Commun., 2209.
[2] S. Künzel, et al. Poster 17 presented at Solid Phase Synthesis & Combinatorial Libraries, Southampton, September 2001.
[3] K. F. Medzihradszky, et al. (2002) Lett. Pept. Sci., 8, 1.
[4] Albericio, et al.Poster 44 presented at American Peptide Symposium, San Diego 2005..
[5] M. Cudic, et al. in ′Peptides 2000, Proc. 26th European Peptide Symposium′, J. Martinez & J.-A. Fehrentz (Eds), Paris, Editions EDK, 2001, pp. 203.
[6] M. Cudic, et al. (2000) Tetrahedron Lett., 41, 4527.
[7] J. P. Malkinson, et al. (2003) Org. Lett., 5, 5051.
Associated Protocols and Technical Articles
Cleavage and Deprotection Protocols for Fmoc SPPS
Literature references
[1] W. C. Chan, et al. (1995) J. Chem. Soc., Chem. Commun., 2209.
[2] S. Künzel, et al. Poster 17 presented at Solid Phase Synthesis & Combinatorial Libraries, Southampton, September 2001.
[3] K. F. Medzihradszky, et al. (2002) Lett. Pept. Sci., 8, 1.
[4] Albericio, et al.Poster 44 presented at American Peptide Symposium, San Diego 2005..
[5] M. Cudic, et al. in ′Peptides 2000, Proc. 26th European Peptide Symposium′, J. Martinez & J.-A. Fehrentz (Eds), Paris, Editions EDK, 2001, pp. 203.
[6] M. Cudic, et al. (2000) Tetrahedron Lett., 41, 4527.
[7] J. P. Malkinson, et al. (2003) Org. Lett., 5, 5051.
결합
Replaces: 04-12-1176
분석 메모
Color (visual): white to slight yellow to beige
Appearance of substance (visual): powder
Identity (IR): passes test
Enantiomeric purity: ≥ 99.0 % (a/a)
Purity (TLC(157A)): ≥ 97 %
Purity (TLC(CMA2)): ≥ 97 %
Assay (HPLC, area%): ≥ 95.0 % (a/a)
Solubility (1 mmole in 2 ml DMF): clearly soluble
Water (K. F.): ≤ 1.0 %
To see the solvent systems used for TLC of Novabiochem® products please click here.
Appearance of substance (visual): powder
Identity (IR): passes test
Enantiomeric purity: ≥ 99.0 % (a/a)
Purity (TLC(157A)): ≥ 97 %
Purity (TLC(CMA2)): ≥ 97 %
Assay (HPLC, area%): ≥ 95.0 % (a/a)
Solubility (1 mmole in 2 ml DMF): clearly soluble
Water (K. F.): ≤ 1.0 %
To see the solvent systems used for TLC of Novabiochem® products please click here.
법적 정보
Novabiochem is a registered trademark of Merck KGaA, Darmstadt, Germany
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
시험 성적서(COA)
제품의 로트/배치 번호를 입력하여 시험 성적서(COA)을 검색하십시오. 로트 및 배치 번호는 제품 라벨에 있는 ‘로트’ 또는 ‘배치’라는 용어 뒤에서 찾을 수 있습니다.
문서
Novabiochem® offers orthogonally protected amino acids for peptide synthesis, including cyclic and branched peptides.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.