G7208
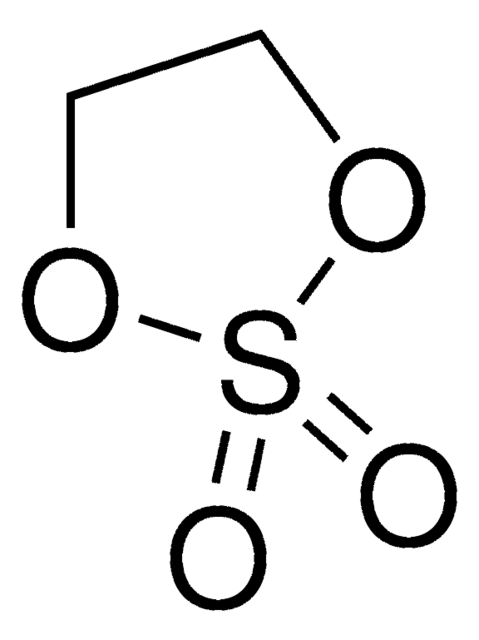
Ethylene sulfite
98%
동의어(들):
1,3,2-Dioxathiolan-2-oxide, Cyclic ethylene sulfite, ES, Glycol sulfite
로그인조직 및 계약 가격 보기
모든 사진(1)
크기 선택
보기 변경
25 G
₩73,385
About This Item
실험식(Hill 표기법):
C2H4O3S
CAS Number:
Molecular Weight:
108.12
Beilstein:
1237109
EC Number:
MDL number:
UNSPSC 코드:
12352100
PubChem Substance ID:
NACRES:
NA.22
추천 제품
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point (°F)
197.1 °F
Flash Point (°C)
91.7 °C
개인 보호 장비
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
이미 열람한 고객
Joseph P O'Shea et al.
European journal of pharmaceutics and biopharmaceutics : official journal of Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik e.V, 96, 207-216 (2015-07-29)
Novel formulations that overcome the solubility limitations of poorly water soluble drugs (PWSD) are becoming ever more critical to a drug development process inundated with these compounds. There is a clear need for developing bio-enabling formulation approaches to improve oral
Dharmendra K Yadav et al.
AAPS PharmSciTech, 16(4), 855-864 (2015-01-15)
The objective of this study was to develop novel docetaxel phospholipid nanoparticles (NDPNs) for intravenous administration. Modified solvent diffusion-evaporation method was adopted in the NDPN preparation. Central composite design (CCD) was employed in the optimization of the critical formulation factor
Hywel D Williams et al.
Journal of pharmaceutical sciences, 103(6), 1734-1746 (2014-04-18)
The current study determined the extent to which the desorption of lipid-based formulations (LBFs) from a mesoporous magnesium aluminometasilicate (Neusilin®-US2) carrier is governed by drug properties, LBF composition, and LBF-to-adsorbent ratio. A secondary objective was to evaluate the impact of
Hywel D Williams et al.
Journal of pharmaceutical sciences, 103(8), 2441-2455 (2014-07-06)
The Lipid Formulation Classification System Consortium looks to develop standardized in vitro tests and to generate much-needed performance criteria for lipid-based formulations (LBFs). This article highlights the value of performing a second, more stressful digestion test to identify LBFs near
Orlagh M Feeney et al.
Journal of controlled release : official journal of the Controlled Release Society, 192, 219-227 (2014-07-25)
For over 20years, stealth drug delivery has been synonymous with nanoparticulate formulations and intravenous dosing. The putative determinants of stealth in these applications are the molecular weight and packing density of a hydrophilic polymer (commonly poly(ethylene glycol) (PEG)) that forms
활성 필터
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.