929379
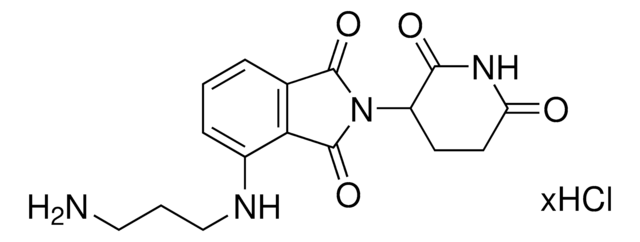
Opto-thalidomide-O-acetamide-C4-NH2 hydrochloride
동의어(들):
4,5-Dimethoxy-2-nitrobenzyl 3-(4-(2-((4-aminobutyl)amino)-2-oxoethoxy)-1,3-dioxoisoindolin-2-yl)-2,6-dioxopiperidine-1-carboxylate hydrochloride
로그인조직 및 계약 가격 보기
모든 사진(1)
About This Item
실험식(Hill 표기법):
C29H31N5O12 · xHCl
Molecular Weight:
641.58 (free base basis)
UNSPSC 코드:
12352101
NACRES:
NA.22
추천 제품
ligand
thalidomide
Quality Level
형태
powder
작용기
amine
저장 온도
2-8°C
SMILES string
COC1=CC(COC(N2C(CCC(N3C(C4=CC=CC(OCC(NCCCCN)=O)=C4C3=O)=O)C2=O)=O)=O)=C(C=C1OC)[N+]([O-])=O.Cl
애플리케이션
Protein degrader building block Opto-thalidomide-O-acetamide-C4-NH2 hydrochloride enables the synthesis of molecules for light-induced targeted protein degradation and PROTAC® (proteolysis-targeting chimeras) research. This conjugate contains a Cereblon (CRBN) recruiting ligand, a rigid linker, and a pendant amine for reactivity with a carboxylic acid on the target ligand. Because even slight alterations in ligands and crosslinkers can affect ternary complex formation between the target, E3 ligase, and degrader, many analogs are prepared to screen for optimal target degradation. When used with other protein degrader building blocks with a terminal amine, parallel synthesis can be used to more quickly generate degrader libraries that feature variation in crosslinker length, composition, and E3 ligase ligand.
Technology Spotlight: Degrader Building Blocks for Targeted Protein Degradation
Protein Degrader Building Blocks
Technology Spotlight: Degrader Building Blocks for Targeted Protein Degradation
Protein Degrader Building Blocks
기타 정보
법적 정보
PROTAC is a registered trademark of Arvinas Operations, Inc., and is used under license
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 4 Oral - Repr. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
시험 성적서(COA)
제품의 로트/배치 번호를 입력하여 시험 성적서(COA)을 검색하십시오. 로트 및 배치 번호는 제품 라벨에 있는 ‘로트’ 또는 ‘배치’라는 용어 뒤에서 찾을 수 있습니다.
Jing Liu et al.
Science advances, 6(8), eaay5154-eaay5154 (2020-03-05)
By hijacking endogenous E3 ligase to degrade protein targets via the ubiquitin-proteasome system, PROTACs (PRoteolysis TArgeting Chimeras) provide a new strategy to inhibit protein targets that were regarded as undruggable before. However, the catalytic nature of PROTAC potentially leads to
Daniel P Bondeson et al.
Annual review of pharmacology and toxicology, 57, 107-123 (2016-10-13)
Protein homeostasis networks are highly regulated systems responsible for maintaining the health and productivity of cells. Whereas therapeutics have been developed to disrupt protein homeostasis, more recently identified techniques have been used to repurpose homeostatic networks to effect degradation of
Momar Toure et al.
Angewandte Chemie (International ed. in English), 55(6), 1966-1973 (2016-01-13)
The current inhibitor-based approach to therapeutics has inherent limitations owing to its occupancy-based model: 1) there is a need to maintain high systemic exposure to ensure sufficient in vivo inhibition, 2) high in vivo concentrations bring potential for off-target side effects, and 3) there is
Kedra Cyrus et al.
Molecular bioSystems, 7(2), 359-364 (2010-10-06)
Conventional genetic approaches have provided a powerful tool in the study of proteins. However, these techniques often preclude selective manipulation of temporal and spatial protein functions, which is crucial for the investigation of dynamic cellular processes. To overcome these limitations
Philipp M Cromm et al.
Cell chemical biology, 24(9), 1181-1190 (2017-06-27)
Traditional pharmaceutical drug discovery is almost exclusively focused on directly controlling protein activity to cure diseases. Modulators of protein activity, especially inhibitors, are developed and applied at high concentration to achieve maximal effects. Thereby, reduced bioavailability and off-target effects can
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.
![3-[1,3-Dihydro-4-(5-hydroxy-1-pentyn-1-yl)-1-oxo-2H-isoindol-2-yl]-2,6-piperidinedione ≥95.0%](/deepweb/assets/sigmaaldrich/product/structures/165/184/ebc29f1b-f63f-4e48-afb5-b3aa4c69795a/640/ebc29f1b-f63f-4e48-afb5-b3aa4c69795a.png)