905348
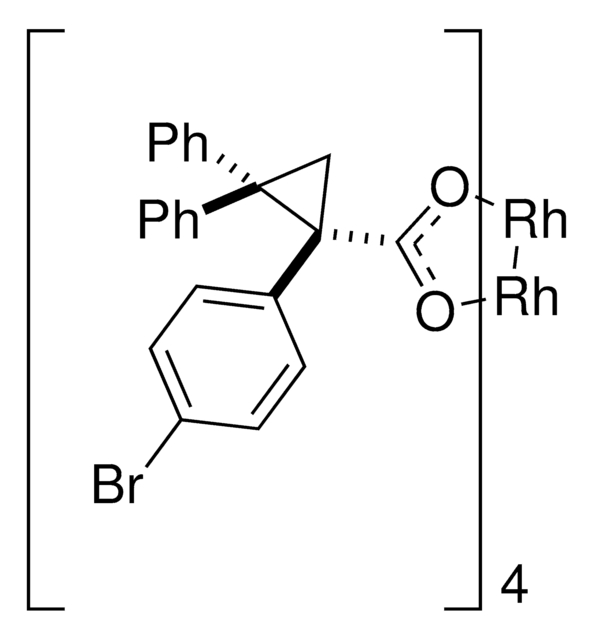
Rh2(R-p-Ph-TPCP)4
동의어(들):
Dirhodium tetrakis((S,R)-1-(4-phenyl(phenyl))-2,2-diphenylcyclopropanecarboxylate), Dirhodium tetrakis[(R)-1-(4-phenyl(phenyl))-2,2- diphenylcyclopropane carboxylate], Tetrakis[(R)-1-(4-phenyl(phenyl))-2,2- diphenylcyclopropane carboxylato]dirhodium(II)
로그인조직 및 계약 가격 보기
모든 사진(3)
About This Item
실험식(Hill 표기법):
C112H84O8Rh2
CAS Number:
Molecular Weight:
1763.67
UNSPSC 코드:
12352101
NACRES:
NA.22
추천 제품
형태
powder
애플리케이션
Rh catalyst developed by the Davies lab used for enantioselective cyclopropanations and C-H functionalization under low catalyst loadings.
기타 정보
Rh2(R-TPCP)4-Catalyzed Enantioselective [3+2]-Cycloaddition between Nitrones and Vinyldiazoacetates
Role of Sterically Demanding Chiral Dirhodium Catalysts in Site-Selective C-H Functionalization of Activated Primary C-H Bonds
Rhodium(II)-Catalyzed C-H Functionalization of Electron-Deficient Methyl Groups
Role of Sterically Demanding Chiral Dirhodium Catalysts in Site-Selective C-H Functionalization of Activated Primary C-H Bonds
Rhodium(II)-Catalyzed C-H Functionalization of Electron-Deficient Methyl Groups
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
시험 성적서(COA)
제품의 로트/배치 번호를 입력하여 시험 성적서(COA)을 검색하십시오. 로트 및 배치 번호는 제품 라벨에 있는 ‘로트’ 또는 ‘배치’라는 용어 뒤에서 찾을 수 있습니다.
Rh2(R-TPCP)4-catalyzed enantioselective [3+2]-cycloaddition between nitrones and vinyldiazoacetates.
Changming Qin et al.
Journal of the American Chemical Society, 135(39), 14516-14519 (2013-09-13)
Rhodium-catalyzed reaction of vinyldiazoacetates with nitrones results in a formal [3+2]-cycloaddition to generate 2,5-dihydroisoxazoles with high levels of asymmetric induction. The cascade reaction begins with a vinylogous addition event, followed by an iminium addition ring-closure/hydride migration/alkene isomerization cascade. Dirhodium tetrakis(triarylcyclopropanecarboxylates)
Liangbing Fu et al.
Journal of the American Chemical Society, 138(18), 5761-5764 (2016-04-12)
Enantioselective C-H functionalization of relatively electron-deficient methyl sites was achieved with the combination of 2,2,2-trichloroethyl aryldiazoacetates and tetrakis(triarylcyclopropanecarboxylate) dirhodium catalysts. The substrate scope of the transformation was relatively broad, and C-H functionalization products were furnished with excellent levels of enantioselectivity.
Changming Qin et al.
Journal of the American Chemical Society, 136(27), 9792-9796 (2014-06-17)
The influence of sterically demanding dirhodium tetracarboxylate catalysts on the site selectivity of C-H functionalization by means of rhodium carbene-induced C-H insertion is described. The established dirhodium tetraprolinate-catalyzed reactions of aryldiazoacetates cause preferential C-H functionalization of secondary C-H bonds as
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.![Bis[rhodium(α,α,α′,α′-tetramethyl-1,3-benzenedipropionic acid)] 95%](/deepweb/assets/sigmaaldrich/product/structures/102/178/d1171a49-0358-406b-8b32-04324dbf9c02/640/d1171a49-0358-406b-8b32-04324dbf9c02.png)