79989
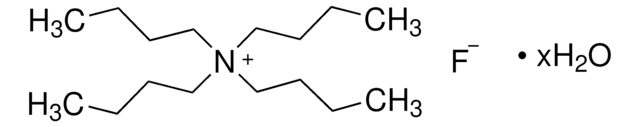
Tetrabutylammonium cyanide
technical, ≥80%
동의어(들):
N,N,N-tributyl-1-butanaminium cyanide
크기 선택
About This Item
추천 제품
grade
technical
Quality Level
분석
≥80%
양식
crystals
mp
89-92 °C (lit.)
SMILES string
[C-]#N.CCCC[N+](CCCC)(CCCC)CCCC
InChI
1S/C16H36N.CN/c1-5-9-13-17(14-10-6-2,15-11-7-3)16-12-8-4;1-2/h5-16H2,1-4H3;/q+1;-1
InChI key
KRRBFUJMQBDDPR-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
관련 카테고리
애플리케이션
- For the deprotection of aliphatic thioacetate to synthesize free thiols in the presence of a protic solvent[1].
- In the O-TMS cyanosilylation of carbonyl compounds to synthesize cyanohydrin trimethylsilyl ethers in the presence of trimethylsilyl cyanide (TMSCN)[2].
- For the ring expansion of β-lactams to synthesize γ-lactams through a bond cleavage of the β-lactam in the presence of acetonitrile[3].
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 1 Dermal - Acute Tox. 2 Inhalation - Acute Tox. 2 Oral - Aquatic Acute 1 - Aquatic Chronic 1
보충제 위험성
Storage Class Code
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
활성 필터
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.