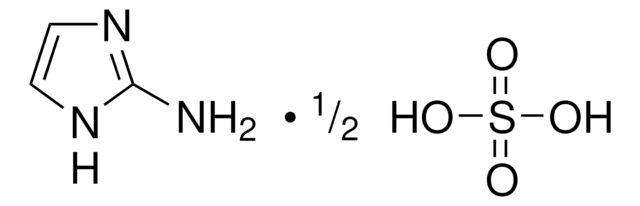추천 제품
분석
97%
mp
93-96 °C (lit.)
작용기
amide
chloro
phenyl
SMILES string
ClCC(=O)NCc1ccccc1
InChI
1S/C9H10ClNO/c10-6-9(12)11-7-8-4-2-1-3-5-8/h1-5H,6-7H2,(H,11,12)
InChI key
SRAXAXHQMCQHSH-UHFFFAOYSA-N
일반 설명
2-Chloro-N-benzylacetamide can be prepared by reacting benzylamine and chloroacetylchloride.[1]
애플리케이션
2-Chloro-N-benzylacetamide may be used in the preparation of:
- 2-[4-(Aryl substituted) piperazin-1-yl]-N-benzylacetamides by reacting with corresponding arylpiperazines.[1]
- N-Methylacetamide and N-benzylacetamide by reacting with di-tert-butyl 1,4,7-triazacyclononane-1,4-diacetate.[2]
- bis[N-Benzyl-2-(quinolin-8-yloxy)acetamide] monohydrate by reacting with 8-hydroxyquinoline.[3]
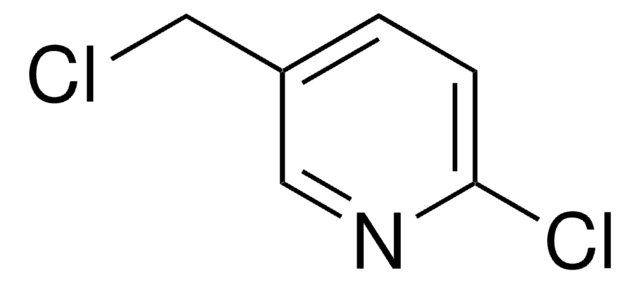
- N-Benzyl-2-(1H-imidazol-1-yl)acetamide by reacting with imidazole.[4]
Storage Class Code
13 - Non Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Gloves, type N95 (US)
가장 최신 버전 중 하나를 선택하세요:
Formation and Characterization of Gallium (III) Complexes with Monoamide Derivatives of 1,4,7-Triazacyclononane-1,4,7-triacetic Acid: A Study of the Dependency of Structure on Reaction pH.
Shetty D, et al.
European Journal of Inorganic Chemistry, 2010(34), 5432-5438 (2010)
Bis [N-benzyl-2-(quinolin-8-yloxy) acetamide] monohydrate.
Wang MS, et al.
Acta Crystallographica Section E, Structure Reports Online, 67(7), o1558-o1558 (2011)
Chiral Variation of a Hybrid Bis (carbene-amido) Ligand System.
Jean-Baptiste dit Dominique F, et al.
Organometallics, 29(13), 2868-2873 (2010)
Synthesis, computational studies and preliminary pharmacological evaluation of 2-[4-(aryl substituted) piperazin-1-yl]-N-benzylacetamides as potential antipsychotics.
Kumar S, et al.
Arabian Journal of Chemistry (2012)
활성 필터
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.