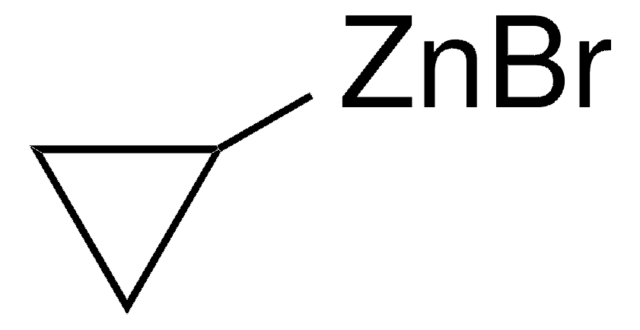
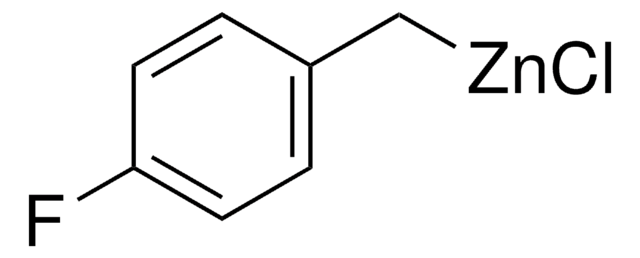
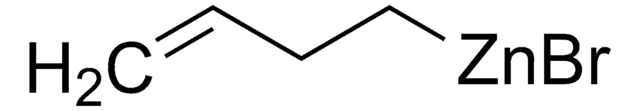
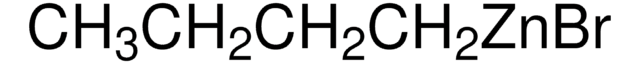모든 사진(1)
About This Item
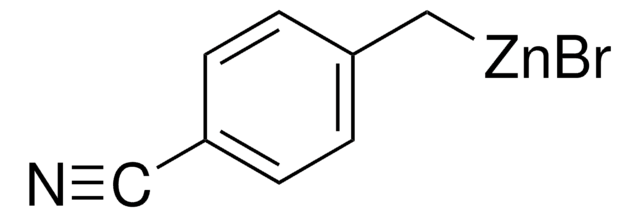
Linear Formula:
NCC6H4CH2ZnBr
CAS Number:
Molecular Weight:
261.43
MDL number:
UNSPSC 코드:
12352103
PubChem Substance ID:
NACRES:
NA.22
추천 제품
농도
0.5 M in THF
density
0.993 g/mL at 25 °C
작용기
nitrile
저장 온도
2-8°C
SMILES string
Br[Zn]Cc1ccccc1C#N
InChI
1S/C8H6N.BrH.Zn/c1-7-4-2-3-5-8(7)6-9;;/h2-5H,1H2;1H;/q;;+1/p-1
InChI key
LQRNYQAXNZETFP-UHFFFAOYSA-M
애플리케이션
2-Cyanobenzylzinc bromide can be used as a reactant:
- In the metal-catalyzed Negishi cross-coupling reactions to prepare aryl or heteroaryl derivatives via carbon-carbon bond formation.
- To synthesize 4-(2-Cyanobenzyl)-3′-(trifluoromethyl)biphenyl by reacting with aryl nonaflate in the presence of Pd(dba)2 as a catalyst.
- To prepare 2-[(2-cyanophenyl)methyl] benzamide by treating with 2-iodobenzamide using a nickel catalyst.
법적 정보
Product of Rieke Metals, Inc.
Rieke is a registered trademark of Rieke Metals, Inc.
Rieke is a registered trademark of Rieke Metals, Inc.
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Carc. 2 - Eye Irrit. 2 - Flam. Liq. 2 - STOT SE 3
표적 기관
Central nervous system, Respiratory system
보충제 위험성
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point (°F)
1.4 °F - closed cup
Flash Point (°C)
-17 °C - closed cup
Improved nickel-catalyzed cross-coupling reaction conditions between ortho-substituted aryl iodides/nonaflates and alkylzinc iodides in solution and in the solid-phase
Jensen AE, et al.
Tetrahedron, 56(25), 4197-4201 (2000)
Visible-Light-Induced Nickel-Catalyzed Negishi Cross-Couplings by Exogenous-Photosensitizer-Free Photocatalysis
Abdiaj I, et al.
Angewandte Chemie (International Edition in English), 57(28), 8473-8477 (2018)
Palladium-catalyzed cross-coupling reactions with aryl nonaflates: a practical alternative to aryl triflates
Rottlander M and Knochel P
The Journal of Organic Chemistry, 63(1), 203-208 (1998)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.