497401
(R)-(+)-2-Methyl-2-propanesulfinamide
98%
동의어(들):
(R)-2-methyl-2-propanesulfinamide, (R)-2-methylpropane-2-sulfinamide, (R)-tert-butanesulfinamide, (R)-tert-butylsulfinamide
About This Item
추천 제품
분석
98%
광학 활성
[α]20/D +4°, c = 1.0242 in chloroform stab. with amylenes
mp
103-107 °C (lit.)
저장 온도
2-8°C
SMILES string
CC(C)(C)S(N)=O
InChI
1S/C4H11NOS/c1-4(2,3)7(5)6/h5H2,1-3H3/t7-/m1/s1
InChI key
CESUXLKAADQNTB-SSDOTTSWSA-N
일반 설명
애플리케이션
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Gloves, type N95 (US)
시험 성적서(COA)
제품의 로트/배치 번호를 입력하여 시험 성적서(COA)을 검색하십시오. 로트 및 배치 번호는 제품 라벨에 있는 ‘로트’ 또는 ‘배치’라는 용어 뒤에서 찾을 수 있습니다.
이미 열람한 고객
문서
Ellman's sulfinamide is available in both enantiomeric and racemic forms for your research. This versatile and useful auxiliary has found extensive use both in academics and industry.
관련 콘텐츠
The Ellman group has participated in the development of a variety of C-H functionalization methods. An electron rich phosphine ligand has proven to be very useful for a variety of Rh(I)-catalyzed C-C bond forming reactions applicable to heterocycle synthesis as exemplified in the recent Science paper “Proton Donor Acidity Controls Selectivity in Nonaromatic Nitrogen Heterocycle Synthesis.” Another useful ligand developed for the highly functional group compatible direct arylation of nitrogen heterocycles is described in a 2008 J. Am. Chem. Soc. paper “Rh(I)-Catalyzed Arylation of Heterocycles via C-H Bond Activation: Expanded Scope through Mechanistic Insight.” The Ellman group also developed the chiral amine reagent tert-Butanesulfinamide, which is extensively used in academics and industry for the asymmetric synthesis of amines. A comprehensive survey of tert-Butanesulfinamide methods and applications up through 2009 is provided in the 2010 Chemical Reviews article, “Synthesis and Applications of tert-Butanesulfinamide.”
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.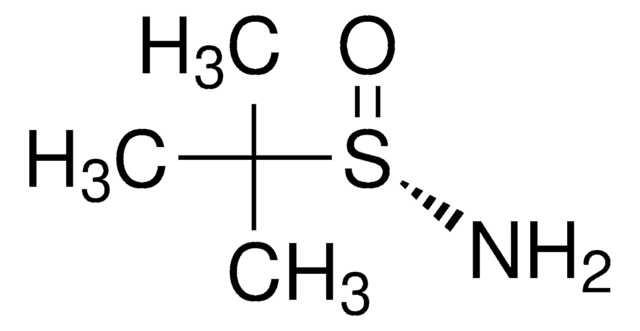








![(R)-N-[(1R,2R)-2-(3-(3,5-Bis(trifluoromethyl)phenyl)ureido)cyclohexyl]-tert-butyl-sulfinamide 96%](/deepweb/assets/sigmaaldrich/product/structures/389/070/18847164-c6a7-4b4e-abcb-2dbc22493a2d/640/18847164-c6a7-4b4e-abcb-2dbc22493a2d.png)