추천 제품
분석
95%
refractive index
n20/D 1.46 (lit.)
bp
80 °C/60 mmHg (lit.)
density
0.966 g/mL at 25 °C (lit.)
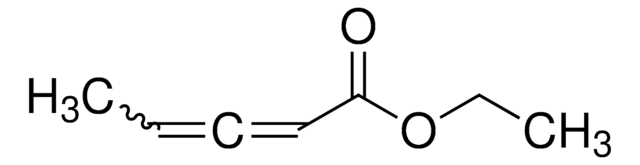
SMILES string
[H]C([H])=C=C([H])C(=O)OCC
InChI
1S/C6H8O2/c1-3-5-6(7)8-4-2/h5H,1,4H2,2H3
InChI key
GLSUOACRAMLJIW-UHFFFAOYSA-N
일반 설명
Ethyl 2,3-butadienoate is an α-allenic ester. The reaction of ethyl 2,3-butadienoate with N-tosylated imines in the presence of DABCO (1,4-diazabicyclo[2.2.2]octane) or DMAP (4-dimethylaminopyridine) forms azetidine derivatives or novel dihydropyridine derivatives respectively. The performance of bifunctional N-acyl aminophosphines to catalyze the asymmetric [3+2] cycloaddition of phenylidenemalononitrile with ethyl 2,3-butadienoate has been evaluated.
애플리케이션
Ethyl 2,3-butadienoate may be used in the synthesis of dihydropyrans by reacting with acyclic enones. It may also be used to synthesize spiranic heterocycles by reacting with heterocyclic bis-arylidene ketones via phosphine-catalyzed [3+2] annulations.
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Flam. Liq. 3
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point (°F)
116.6 °F - closed cup
Flash Point (°C)
47 °C - closed cup
개인 보호 장비
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
시험 성적서(COA)
제품의 로트/배치 번호를 입력하여 시험 성적서(COA)을 검색하십시오. 로트 및 배치 번호는 제품 라벨에 있는 ‘로트’ 또는 ‘배치’라는 용어 뒤에서 찾을 수 있습니다.
이미 열람한 고객
Abnormal aza-Baylis-Hillman reaction of N-tosylated imines with ethyl 2,3-butadienoate and penta-3,4-dien-2-one.
Zhao GL, et al.
Organic Letters, 5(24), 4737-4739 (2003)
Asymmetric [3+2] Cycloadditions of Allenoates and Dual Activated Olefins Catalyzed by Simple Bifunctional N-Acyl Aminophosphines.
Xiao H, et al.
Angewandte Chemie (International Edition in English), 49(26), 4467-4470 (2010)
Heterocyclic Spiranes and Dispiranes via Enantioselective Phosphine-Catalyzed [3+2] Annulations.
Duvvuru D, et al.
Advanced Synthesis & Catalysis, 354(2-3), 408-414 (2012)
Development of a formal catalytic asymmetric [4+2] addition of ethyl-2,3-butadienoate with acyclic enones.
Ashtekar KD, et al.
Organic Letters, 13(21), 5732-5735 (2011)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.