모든 사진(2)
크기 선택
보기 변경
5 G
₩101,399
25 G
₩222,590
About This Item
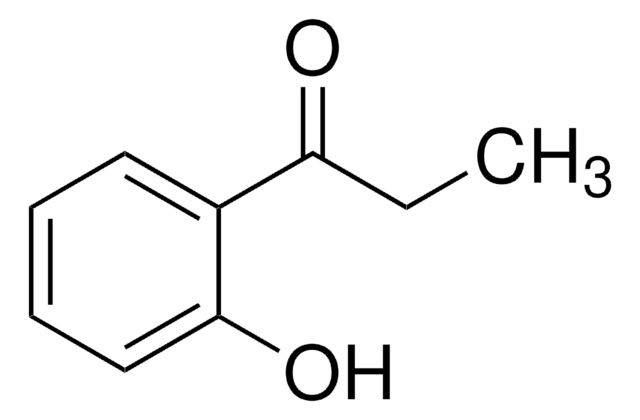
Linear Formula:
C6H5COCH2OH
CAS Number:
Molecular Weight:
136.15
EC Number:
MDL number:
UNSPSC 코드:
12352100
PubChem Substance ID:
NACRES:
NA.22
추천 제품
Quality Level
분석
98%
mp
86-89 °C (lit.)
작용기
hydroxyl
ketone
phenyl
SMILES string
OCC(=O)c1ccccc1
InChI
1S/C8H8O2/c9-6-8(10)7-4-2-1-3-5-7/h1-5,9H,6H2
InChI key
ZWVHTXAYIKBMEE-UHFFFAOYSA-N
애플리케이션
2-Hydroxyacetophenone can be used as a starting material for the synthesis of:
- Enantioselective 1R-phenyl-1,2-ethanediol in the presence of a rhodium(III) catalyst by asymmetric transfer hydrogenation.[1]
- Copper(II) complexes of 2-hydroxyacetophenone N-substituted thiosemicarbazones.[2]
- Chromium, molybdenum, and ruthenium complexes of 2-hydroxyacetophenone Schiff bases.[3]
- 2-Hydroxyacetophenone-aroyl hydrazone derivatives for inhibition of copper corrosion in nitric acid.[4]
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
표적 기관
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
dust mask type N95 (US), Eyeshields, Gloves
이미 열람한 고객
Resonance Raman intensity analysis of the excited-state proton transfer in 2-hydroxyacetophenone.
Peteanu LA and Mathies RA.
The Journal of Physical Chemistry, 96(17), 6910-6916 (1992)
Chromium, molybdenum and ruthenium complexes of 2-hydroxyacetophenone Schiff bases
Ali SA, et al.
Journal of Coordination Chemistry, 55(10), 1161-1170 (2002)
New copper (II) complexes of 2-hydroxyacetophenone N (4)-substituted thiosemicarbazones and polypyridyl co-ligands: structural, electrochemical and antimicrobial studies
John RP, et al.
Polyhedron, 23(16), 2549-2559 (2004)
Na Li et al.
Molecules (Basel, Switzerland), 25(14) (2020-07-28)
Fluorophores with aggregation-induced emission enhancement (AIEE) characteristics applied in bioimaging have attracted more and more attention in recent years. In this work, a series of flavanone compounds with AIEE characteristics was developed and applied to fluorescence imaging of mitochondria and
Synthetic studies on optically active Schiff-base ligands derived from condensation of 2-hydroxyacetophenone and chiral diamines.
Gao WT and Zheng Z.
Molecules (Basel), 7(7), 511-516 (2002)
활성 필터
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.