모든 사진(1)
크기 선택
보기 변경
25 G
₩396,358
About This Item
Linear Formula:
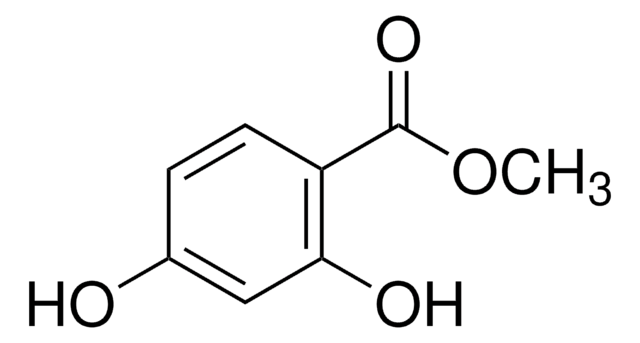
(HO)2C6H3CO2CH3
CAS Number:
Molecular Weight:
168.15
EC Number:
MDL number:
UNSPSC 코드:
12352100
PubChem Substance ID:
NACRES:
NA.22
추천 제품
분석
99%
mp
86-88 °C (lit.)
작용기
ester
SMILES string
COC(=O)c1cc(O)ccc1O
InChI
1S/C8H8O4/c1-12-8(11)6-4-5(9)2-3-7(6)10/h2-4,9-10H,1H3
InChI key
XGDPKUKRQHHZTH-UHFFFAOYSA-N
관련 카테고리
일반 설명
Methyl 2,5-dihydroxybenzoate (methyl gentisate) is an alkyl ester of gentisic acid. It is reported to show less cytotoxic and mutagenic activity than hydroquinone with a potential to inhibit melanogenesis.[1] It has been synthesized from 2,5-dihydroxybenzoic acid. The crystal structure of the molecule was found to be planar.[2]
애플리케이션
Methyl 2,5-dihydroxybenzoate (methyl gentisate) may be used as a starting material in the synthesis of euonyminol.[3]
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
표적 기관
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
dust mask type N95 (US), Eyeshields, Gloves
가장 최신 버전 중 하나를 선택하세요:
시험 성적서(COA)
Lot/Batch Number
Methyl 2, 5-dihydroxybenzoate.
Brown CL, et al.
Acta Crystallographica Section E, Structure Reports Online, 59(5), 630-631 (2003)
E V Curto et al.
Biochemical pharmacology, 57(6), 663-672 (1999-02-26)
To discover safe and effective topical skin-lightening agents, we have evaluated alkyl esters of the natural product gentisic acid (GA), which is related to our lead compound methyl gentisate (MG), and four putative tyrosinase inhibitors, utilizing mammalian melanocyte cell cultures
Total Synthesis of (.+-.)-Euonyminol, the Sesquiterpenoid Nucleus of Cathedulin K-19, via an Epoxide Cascade Cyclization.
White JD, et al.
Journal of the American Chemical Society, 117(38), 9780-9781 (1995)
활성 필터
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.