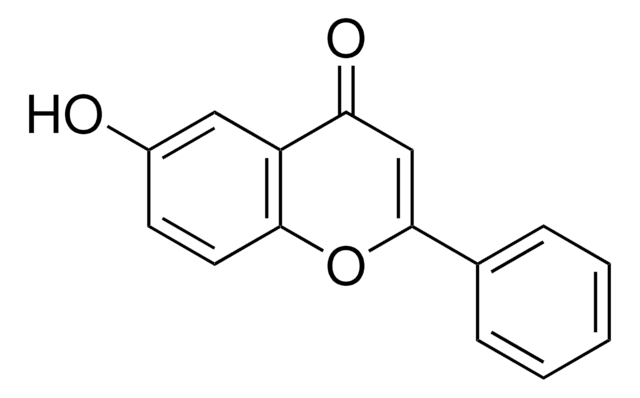
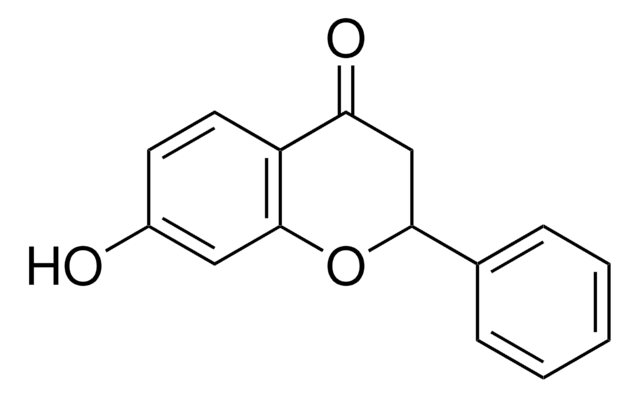
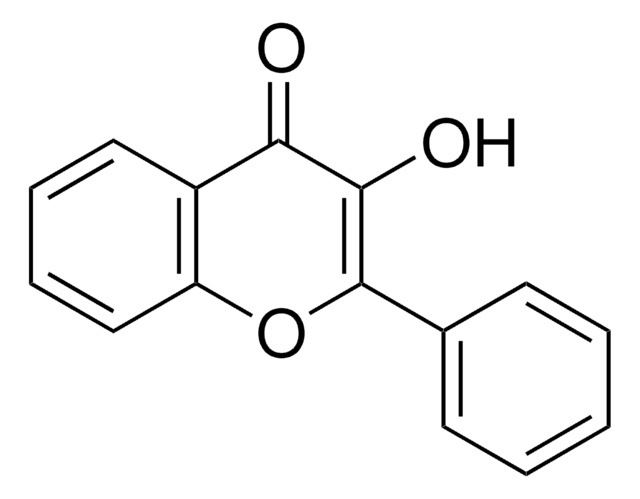
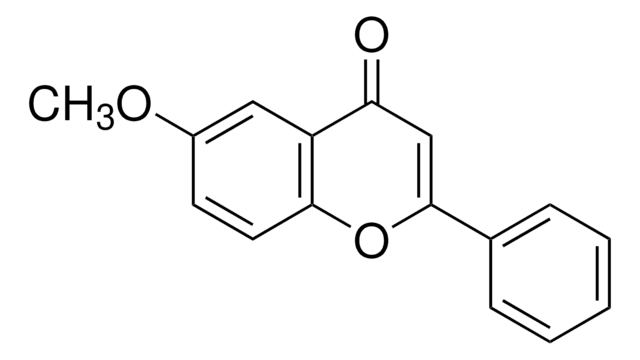
추천 제품
일반 설명
6-Hydroxyflavanone is a flavonoid. Its biotransformation by Aspergillus niger strains have been described. Crystal structure of S and R enantiomers of 6-hydroxyflavanone has been reported to have four crystallographic sites of the unit cell in an approximate 3:1/1:3 ratio. It was identified as a metabolite of flavnone on incubation with rat liver microsomes by positive ion electrospray LC/MS analysis.
애플리케이션
6-Hydroxyflavanone (6-HF) has been used for the enantiomeric separation of flavonoids using polysaccharide-based chiral stationary phases by nano-liquid chromatography (nano-LC). Racemic 6-HF may be used in the synthesis of 6-propionoxy-flavanone (6-PF). 6-HF may be employed as synthetic flavone to investigate the mechanism of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) induced cytotoxic and apoptotic effects in HeLa cancer cells.
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
표적 기관
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
dust mask type N95 (US), Eyeshields, Gloves
시험 성적서(COA)
제품의 로트/배치 번호를 입력하여 시험 성적서(COA)을 검색하십시오. 로트 및 배치 번호는 제품 라벨에 있는 ‘로트’ 또는 ‘배치’라는 용어 뒤에서 찾을 수 있습니다.
Disordered 6-hydroxyflavanone.
Bialonska A, et al.
Acta Crystallographica Section E, Structure Reports Online, 63(2), 430-431 (2007)
Ewelina Szliszka et al.
Molecules (Basel, Switzerland), 17(10), 11693-11711 (2012-10-03)
Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) is considered as the most promising anticancer agent in the TNF superfamily because of its selective cytotoxicity against tumor cells versus normal primary cells. However, as more tumor cells are reported to be resistant
Dejan Nikolic et al.
Drug metabolism and disposition: the biological fate of chemicals, 32(4), 387-397 (2004-03-25)
Flavonoids represent a diverse group of natural pigments widely distributed in the plant kingdom and are an important component of human diet due to their high content in fruits and vegetables. Since many flavonoids have been shown to be potent
Microbial transformations of flavanone and 6-hydroxyflavanone by Aspergillus niger strains.
Kostrzewa-Suslow E, et al.
Journal of Molecular Catalysis. B, Enzymatic, 39(1), 18-23 (2006)
Kahina Si-Ahmed et al.
Analytica chimica acta, 738, 85-94 (2012-07-14)
Three polysaccharide-based chiral stationary phases, Sepapak(®) 1, Sepapak(®) 2 and Sepapak(®) 3 have been evaluated in the present work for the stereoisomer separation of a group of 12 flavonoids including flavanones (flavanone, 4'-methoxyflavanone, 6-methoxyflavanone, 7-methoxyflavanone, 2'-hydroxyflavanone, 4'-hydroxyflavanone, 6-hydroxyflavanone, 7-hydroxyflavanone, hesperetin
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.